



版权说明:本文档由用户提供并上传,收益归属内容提供方,若内容存在侵权,请进行举报或认领
文档简介
1、【鹵烷反應,Reaction of Haloalkanes】(一)親核取代反應Nucleophilic substitution Reaction.鹵烷的典型反應為親核取代反應。鹵烷內的碳鹵鍵被極化,使碳原子帶有少許正電荷,故鹵烷可視為反應中的親電子體。The typical reaction of a haloalkane is nucleophilic substitution. The carbon-halogen bond in the haloalkane is polarized with a small positive charge on the carbon atom and
2、 so in the reaction, the haloalkane acts as an electrophile.在親核取代反應中,親核體攻擊鹵烷的C+ 並置換出一個鹵離子。故若使用不同的親核體,便能產生很多不同的化合物。因此在合成反應中,鹵烷的親核取代反應佔了很重要的位置。當鹵基分別被OH、OR、CH3COO、CN及NH2等基團取代,便能製備出醇、醚、酯、氰化物及胺。In nucleophilic substitution, a nucleophile attacks the C+ and displaces a halide ion from the haloalkane. A la
3、rge variety of compounds can be formed depending on the nucleophile used in the reaction. The nucleophilic substitution of haloalkanes is a very important type of synthetic reaction. This reaction can be applied in the preparation of alcohols, ethers, esters, nitrides and amines when substitution oc
4、curs with byOH, OR, CH3COO, CN and NH2 groups respectively.(A)鹵烷的水解Hydrolysis of Haloalkanes鹵烷能進行水解,生成鹵醇和鹵離子。Haloalkanes can undergo hydrolysis and give alcohol and halide ion as products.HaloalkaneNucleophile(a)與水(OH)反應React with Water(OH)1,一級及二級鹵烷只能與水有輕微反應The reaction with the primary and secondar
5、y haloalkanes are less reactive由於水分子的氧原子帶有微弱負電荷()及兩對孤偶電子,所以水能夠在化學反應中作為親核體。然而,水分子只是一個很弱的親核體,並只能與一級及二級鹵烷有輕微反應。The oxygen atom in the water molecule bears a slightly negative charge() and two lone pairs of electrons, so it functions as a nucleophile in the chemical reactions. However, the water molecul
6、e is only a weak nucleophile and the reaction with the primary and secondary haloalkanes are less reactive unless an alkaline medium ( i.e. a stronger nucleophile, the OH is present) is provided.Reflux2,三級鹵烷,在室溫室壓下已經能夠與水反應至於三級鹵烷,在室溫室壓下已經能夠與水反應。三級醇通常由相對的鹵烷與水進行加熱回流而製得。The tertiary haloalkane is the mo
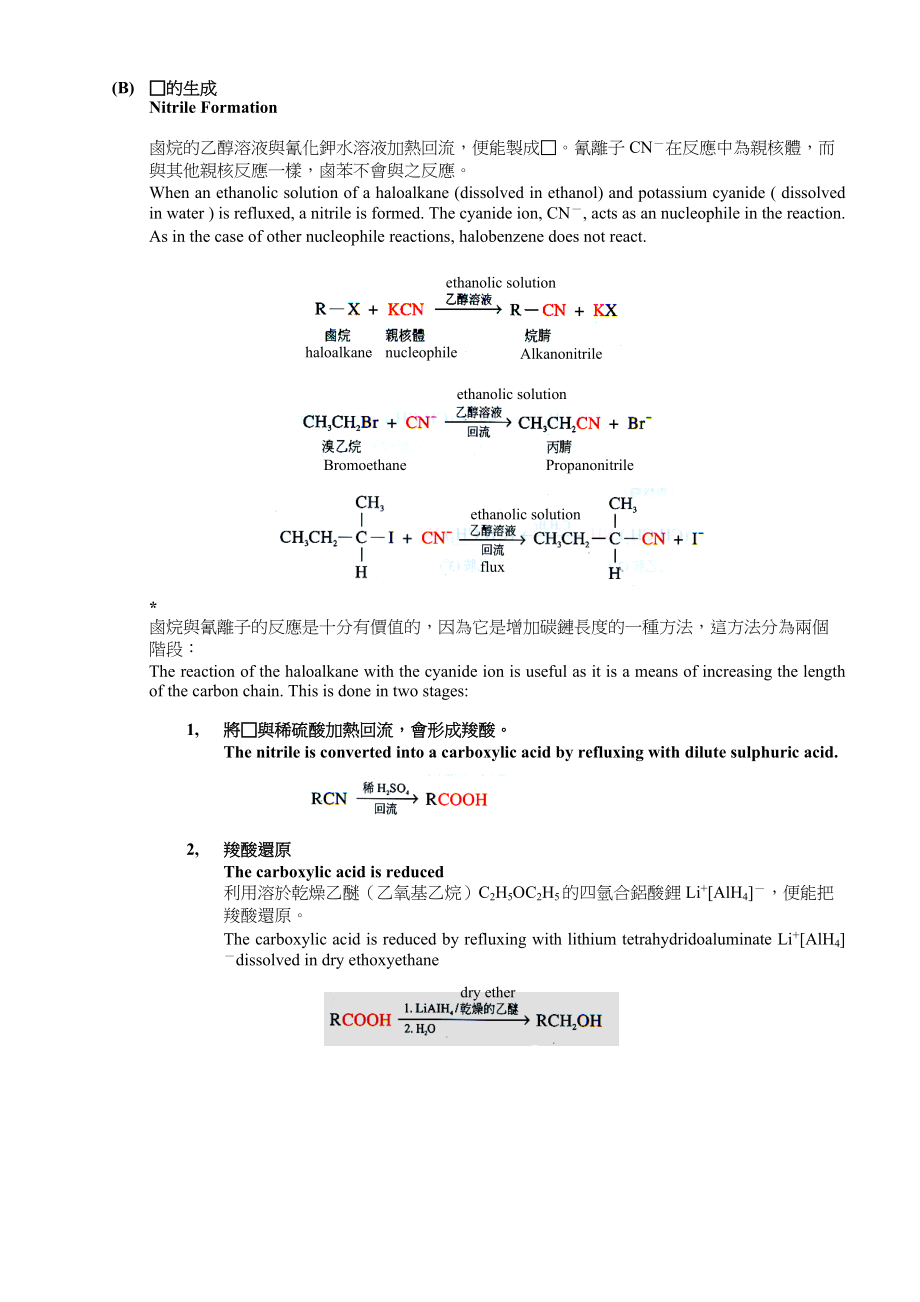
7、st reactive one and reacts readily with water at room condition.(b)與鹼(OH)反應React with the alkaline 在鹼性環境(即強親核體OH)下,一級及二級鹵烷會變得活潑。主要反應會是消去反應。If an alkaline medium is used, elimination becomes the major reaction.(B)的生成Nitrile Formation鹵烷的乙醇溶液與氰化鉀水溶液加熱回流,便能製成。氰離子CN在反應中為親核體,而與其他親核反應一樣,鹵苯不會與之反應。When an et
8、hanolic solution of a haloalkane (dissolved in ethanol) and potassium cyanide ( dissolved in water ) is refluxed, a nitrile is formed. The cyanide ion, CN, acts as an nucleophile in the reaction. As in the case of other nucleophile reactions, halobenzene does not react.haloalkanenucleophileAlkanonit
9、rileethanolic solutionethanolic solutionBromoethanePropanonitrileethanolic solutionflux*鹵烷與氰離子的反應是十分有價值的,因為它是增加碳鏈長度的一種方法,這方法分為兩個階段:The reaction of the haloalkane with the cyanide ion is useful as it is a means of increasing the length of the carbon chain. This is done in two stages:1,將與稀硫酸加熱回流,會形成羧酸
10、。The nitrile is converted into a carboxylic acid by refluxing with dilute sulphuric acid.2,羧酸還原The carboxylic acid is reduced利用溶於乾燥乙醚(乙氧基乙烷)C2H5OC2H5的四氫合鋁酸鋰Li+AlH4,便能把羧酸還原。The carboxylic acid is reduced by refluxing with lithium tetrahydridoaluminate Li+AlH4dissolved in dry ethoxyethanedry ether(C)胺
11、的生成Amine Formation(a)一級(1)胺A primary (1) amine氨的氮原子擁有一對孤偶電子,故它亦是親核體。鹵烷與溶於乙醇的氨在密封的試管(以製造高壓內加熱,便生成胺。由於只有一個烷基與氮原子相連,故乙胺為一級(1)胺。Ammonia is a nucleophile because of the presence of a lone pair of electrons on the nitrogen atom. When a haloalkane and ammonia dissolved in ethanol and heated in a sealed tub
12、e ( to create a high pressure), an amine is formed. As there is only one alkyl group attached to the nitrogen atom, ethylamine is termed a primary (1) amine.ethanolHeat in a sealed tubeHaloalkaneAlkylamineethanolHeat in a sealed tubeBromoethaneEthylamine(b)二級(2)及三級(3)胺Secondary(2) and tertiary(3) am
13、ines二級(2)胺與三級(3)胺則分別有兩個及三個烷基與氮原子相連。如氮一般,胺的氮原子亦有一對孤偶電子,故亦可作為親核體。當氨與過量溴乙烷反應,便會發生進一步的取代反應,並生成二級胺與三級胺的混合物及季銨鹽。For secondary (2) and tertiary (3) amines, there are two and three alkyl groups attached to the nitrogen atom respectively. Similar to ammonia, an amine has a lone pair of electrons on the nitro
14、gen atom and can act as a nucleophile with excess bromoethane, further substitution occurs. A mixture of (2) and tertiary (3) amines, and a quaternary (4) ammonium salt will be formed.EthylamineDiethylamineDiethylamineTriethylamineTetraethylammonium bromide*實際上,所生成的混合物是很難分離的。然而,若利用過量的氨,便可得到較佳的一級胺產量。
15、這個反應一般不會應用在製備上,而二級胺與三級胺更不會以此方法來製備。至於鹵苯在近似的條件下,是不會與氨或胺有任何反應的。In practice, a mixture of products is obtained and such products are hard to separate, but excess of ammonia enables a better yield of the lamine. This reaction is thus of little use in preparating a primary amine. Secondary and tertiary am
16、ines are never made in this way. Halobenzenes do not react with ammonia or amines under similar conditions.(二)消去反應Elimination reaction鹵烷亦能進行鹵代氫的消去反應而生成烯。在烯的消去反應中,會有炔烴的生成。由於取代反應與消去反應均由鹼性的多電子試劑所引發,所以這兩種反應均恆常地競賽。Haloalkanes may undergo elimination of the hydrogen halide to form an alkene. An alkyne can
17、 be formed from the elimination reaction of a alkene. Both elimination and substitution are brought about by basic, electron-rich reagents. Hence there is always competition between the two types of reactions. (A)消去反應只可發生在有氫的鹵烷上(H與CX相鄰的碳連結)Elimination can only occur if the haloalkane has the -hydrog
18、en ( hydrogen attached to the -carbon which is next to CX )(B)鹵烷與溶於乙醇的氫氧化鈉溶液作用,生烯Haloalkane reflux with a solution of sodium hydroxide in ethanol, form alkene(a)反應Reaction在某些情況下,OH會作為鹼多於作為親核體,故它能將鹵烷的H+移離。當OH從CBr相鄰的碳原子上移離H+的同一時間,CBr鍵合便會斷裂。例如,當我們以溶於乙醇的氫氧化鈉溶液替代水,並與2-溴丙烷加熱回流,便會生成丙烯。使用極性較低的溶劑(乙醇),可減少氫氧離子
19、的溶合,並且加強它的推電子能力(或鹼性)。Under certain conditions, OH can act as a base instead of a nucleophile. When OH behaves in this way, it removes H+ from a haloalkane. The CBr bond breaks at the same time as OH removes H+ from its neighbouring carbon atom and an alkene is formed. For example, when 2-bromopropan
20、e is refluxed with a solution of sodium hydroxide in ethanol instead of in water, propene is formed. The use of a less polar solvent (ethanol) decreases the solvation of the hydroxide ion and thus increases its electron-donating ability or basic strength.(b)有利於消去反應的條件Conditions that favour the elimi
21、nation reaction在取代反應中,OH會作為親核體;而在消去反應中,它則會作為鹽基。透過反應條件的控制,可以決定氫氧離子的角色。有利於消去反應的條件,包括:In the substitution reaction OH acts as a nucleophile; while in the elimination reaction it acts as a base. By altering the reaction conditions, we can alter the manner the hydroxide ion acts. Conditions that favour t
22、he elimination reaction include:1,被高度取代的鹵烷a highly substituted haloalkane2,極性較低的溶劑a less polar solvent3,強鹽基作為攻擊物種a strong base acting as the attacking species, and4,高溫high temperture事實上,消去反應與取代反應在這組條件下皆會發生,我們只是控制主反應。Actually, both substitution and elimination will occur under each set of conditions.
23、 we can only control the major type of reaction.(c)依切夫規則The Saytzeff rule根據札依切夫規則,被高度取代的烯烴較穩定,因此為主生成物。Elimination can proceed to give a mixture of alkenes. According to the Saytzeff rule, the highly substituted alkenes are more stable and hence predominate.(d)烯烴的穩定性The stability of alkenes烯烴的穩定性按序為:
24、The stability of alkenes follow this order:(e)炔烴的生成Produces an alkyne二鹵烷經消去反應後,會生成炔烴,而兩個鹵化氫分子亦會在反應中消去。The elimination reaction of a dihaloalkane produces an alkyne. Two molecules of the hydrogen halide are eliminated in the reaction.KOH in ethanolKOH in ethanol【鹵苯反應,Reaction of Halobenzenes】(一)親核取代反
25、應Nucleophilic substitution reactions鹵苯對親核取代反應相對地並不活潑,這種不活潑性與其結構有關。Halobenzenes are comparatively unreactive in nucleophilic substitution reactions. The low reactivity is related to the structure of the compound.(A)離域鍵系統(共振結構)A delocalized bond system ( resonance strucure )在苯環的碳原子上的p軌態與在鹵原子上的p軌態,以側向重
26、疊,並組成一個離域鍵系統(共振結構)。然而,若環中有推電子團如NO和CN的存在,便有利鹵原子的親核取代反應。於是,電子在整個苯環及鹵原子發生離域作用,並會導致The p-orbitals on the carbon atoms of the benzene ring and that on the halogen atom overlap sideways to form a delocalized bond system ( resonance strucure ) . However nucleophilic substitution of the halogen atom may be
27、promoted by the presence of electron withdrawing groups, e.g. NO andCN in the ring. The consequences of the delocalization of -electrons throughout the benzene ring and the halogen atom are:(a)碳鹵鍵被鍵性質強化The carbon-halogen bond is strengthened by its partial -bond character在這情況下,若要使這個鍵斷裂,便需要很大的能量。故此,取代反應變得更加困難。the carbon-halogen bond is strengthened by its partial -bond character. The breakage of the b
温馨提示
- 1. 本站所有资源如无特殊说明,都需要本地电脑安装OFFICE2007和PDF阅读器。图纸软件为CAD,CAXA,PROE,UG,SolidWorks等.压缩文件请下载最新的WinRAR软件解压。
- 2. 本站的文档不包含任何第三方提供的附件图纸等,如果需要附件,请联系上传者。文件的所有权益归上传用户所有。
- 3. 本站RAR压缩包中若带图纸,网页内容里面会有图纸预览,若没有图纸预览就没有图纸。
- 4. 未经权益所有人同意不得将文件中的内容挪作商业或盈利用途。
- 5. 人人文库网仅提供信息存储空间,仅对用户上传内容的表现方式做保护处理,对用户上传分享的文档内容本身不做任何修改或编辑,并不能对任何下载内容负责。
- 6. 下载文件中如有侵权或不适当内容,请与我们联系,我们立即纠正。
- 7. 本站不保证下载资源的准确性、安全性和完整性, 同时也不承担用户因使用这些下载资源对自己和他人造成任何形式的伤害或损失。
最新文档
- 2023九年级数学上册 第25章 随机事件的概率25.2 随机事件的概率 3列举所有机会均等的结果教学实录 (新版)华东师大版
- 小朋友国防教育
- 2025江西专升本《艺术概论》模拟练习试题(附答案)
- 部编版三年级语文上册第二单元第4课《古诗三首》课件
- 蠡县中学高一月月考语文试题
- 学校艾滋病宣传活动总结
- 乡镇卫生院年终工作总结
- 年轻干部廉洁教育
- 生物工程公司特约经销商合同
- 广告委托加工宣传合同
- 房屋建筑工程 危险性较大分部分项工程巡检记录表
- 预防校园欺凌主题班会课件(共36张课件)
- 2024智慧水电厂评价项目表
- 超星尔雅学习通《工程伦理》章节测试答案
- 人工智能通识 课件 04 驾驭AIGC提示词工程(Prompt)
- DB3301-T 65.11-2024 反恐怖防范系统管理规范 第11部分:医院
- T-CPQS C010-2024 鉴赏收藏用潮流玩偶及类似用途产品
- 110kV变电站专项电气试验及调试方案
- 选煤厂安全规程-编辑说明
- 物联网系统安装与调试活页式教程中职全套教学课件
- GB/T 3428-2024架空导线用镀锌钢线

评论
0/150
提交评论