



版权说明:本文档由用户提供并上传,收益归属内容提供方,若内容存在侵权,请进行举报或认领
文档简介
1、半导体光电材料基础半导体光电材料基础ReferencesTexts: P. Bhattacharya, Semiconductor Optoelectronic Devices, 2nd edition, Prentice Hall, 1997. 孟庆巨等,半导体器件物理(第2版),科学出版社,2009 刘恩科等,半导体物理学(第7版),电子工业出版社,2011Additional references: S. O. Kasap, Optoelectronic and Photonics: Principles and Practices, Prentice Hall, 2011. , 199
2、8 黄昆原著,固体物理学,高等教育出版社,1988 半导体光电材料基础考核方式: 分为5个小组,每组3-4人,1人为组长。 每次课的最后两节,由一个小组的组员轮流讲解部分PPT课件,并回答同学和老师的提问。课件内容由老师指定,组内分工合作,可参考中文版本课件准备,但讲解时需采用英文版本课件,使用中文或英文讲解均可。 评分标准:PPT讲解及回答问题 60% 小组内表现 20%(组内成员互相排序) 提问 20% (给讲PPT的同学提问,至少4次) 小组1:非平衡载流子、PN结 (中文版本课件请参考E-learning 2012-2013年度第一学期课件中的第二个课件“半导体物理基础、PN结”)半导
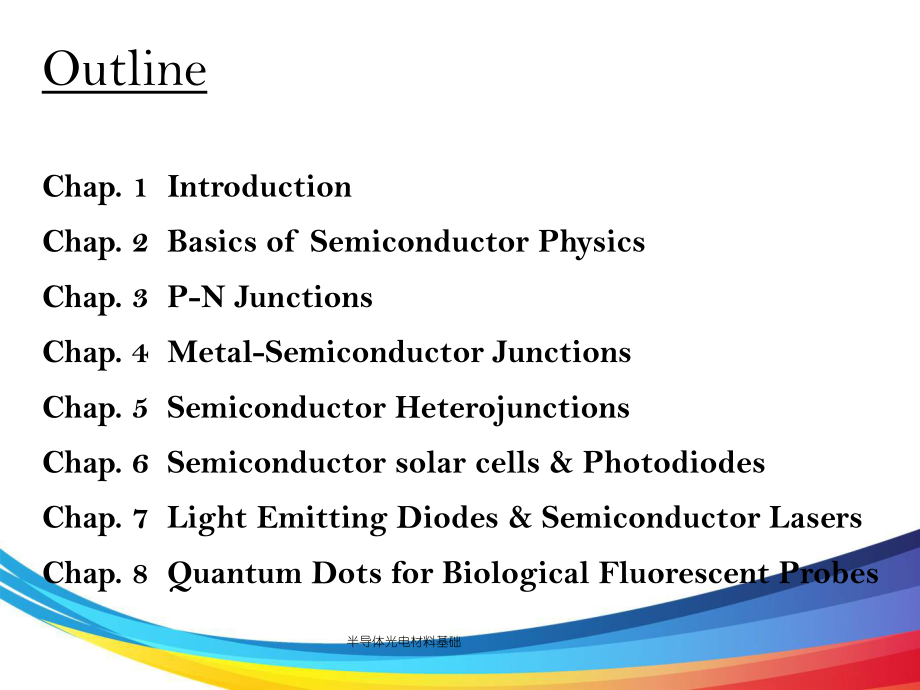
3、体光电材料基础OutlineChap. 1 Introduction Chap. 2 Basics of Semiconductor PhysicsChap. 3 P-N JunctionsChap. 4 Metal-Semiconductor JunctionsChap. 5 Semiconductor HeterojunctionsChap. 6 Semiconductor solar cells & PhotodiodesChap. 7 Light Emitting Diodes & Semiconductor LasersChap. 8 Quantum Dots for
4、 Biological Fluorescent Probes半导体光电材料基础Chapter 1Introduction半导体光电材料基础1.1 Semiconductor Optoelectronic Devices and Materials Semiconductor optoelectronic devices: Semiconductor functional devices in which the interaction of electronic processes with light and optical processes can suitably take place
5、, usually accompanied by an energy conversion process (e.g., form electrical to optical, and vice). Common semiconductor optoelectronic devices: Optical Electrical: Photodetectors, solar cells. Electrical Optical: Light emitting diodes, injection lasers. Optical Optical: Optically-pumped semiconduct
6、or lasers, fluorescent quantum dos. Semiconductor optoelectronic materials: Semiconductor materials used for making the above devices. 半导体光电材料基础1.2 Common Semiconductor Optoelectronic MaterialsSemiconductor Optoelectronic MaterialsIV or IV-IV compoundsGeIII-V compoundsII-VI compoundsIV-VI compoundsS
7、iSiCGaAs; GaP; GaNZnS; ZnO; ZnSe; ZnTeInAs; InP; InSbAlAs; AlPGaAs1-xPx ;In1-xGaxPCdS; CdSe; CdTeHgS; HgSePbS; PbSe; PbSnTe; PbSnSe半导体光电材料基础半导体光电材料基础半导体光电材料基础半导体光电材料基础半导体光电材料基础1.3 Application of Some Semiconductor Optoelectronic Materials Germanium (Ge): Photodetectors. Silicon (Si): Photodetectors,
8、 solar cells. Silicon Carbide (SiC): Electroluminescent devices. Gallium Arsenide (GaAs): Lasers, light emitting diodes (LEDs), solar cells. Zinc Sulfide/Oxide (ZnS, ZnO): Electroluminescent and photoluminescent probes. Cadmium Sulfide (CdS): Solar cells, lasers. Cadmium Telluride (CdTe): Solar cell
9、s, IR photodetectors, fluorescent probes/bioimaging. Lead Sulfide/Selenide/Telluride (PbS, PbSe, PbTe): Solar cells, IR photodetectors, lasers.半导体光电材料基础 The band gap energy normally determines the working wavelength of a semiconductor optoelectronic device. The conversion between electrical and opti
10、cal energies usually relates to the electron transitions between the conduction band and valence band of a semiconductor.1.4 Physics of Semiconductor Opto-electronic Devices半导体光电材料基础 Solar cells, optodetectors LEDs, semiconductor lasers Photoluminescent quantum dots for fluorescent probes1.4 Physics
11、 of Semiconductor Opto-electronic DevicesAbsorption, photoconductive effect, photovoltaic effect Carrier injection, radiative recombinationAbsorption, radiative recombination半导体光电材料基础Chapter 2Basics of Semiconductor Physics半导体光电材料基础2.1 Electron states in One Atom2.2 Electron states and Band Structur
12、es in Semiconductors2.3 Impurity and Defect Levels2.4 Carrier Distribution in a Semiconductor2.5 Conduction Processes in Semiconductors2.6 Non-equilibrium Charge Carriers2. Basics of Semiconductor Physics半导体光电材料基础2.1 Electron states in One Atom2.2 Electron states and Band Structures in Semiconductor
13、s2.3 Impurity and Defect Levels2.4 Carrier Distribution in a Semiconductor2.5 Conduction Processes in Semiconductors2.6 Non-equilibrium Charge Carriers2. Basics of Semiconductor Physics半导体光电材料基础2.1 Electron States in one atomuThe Earlier Proposal of Quantum Theoryu Probability Waveu Schrdinger Equat
14、ionuEnergy Eigenvalue and Eigenfunction of the Hydrogen Atom Modelu Electron Configuration半导体光电材料基础The Earlier Proposal of Quantum TheorylIn 1900, Planck firstly proposed a hypothesis of “quantum”, and derived the blackbody formula, which perfectly matched the observation results. Discrete energy of
15、 electromagnetic radiationlIn 1905, Einstein tried to explain the difficulties in the experiment of photoelectric effect, and put forward the concept of light quantum. Light is particulate.l19121913, Bohr proposed the quantum theory of atomic structure, which successively explained the line spectrum
16、 of hydrogen atom. Discrete energy levels and quantum transition, EhphHaving limitations and problems!半导体光电材料基础The Duality of Matter ParticleslWave-particle duality of light: Wavelike behavior: interference, diffraction, and polarization Particulate nature: blackbody radiation and photoelectric effe
17、ctlde Broglie wave (1924): In the atomic world, matter particles (electrons, protons, neutrons, atoms, etc) have wavelike properties, which have been verified by experiments, i.e., diffraction of electron beams by single crystals.lWave mechanics of Schrdinger (1926) revealing the laws of particle mo
18、vement in the microscopic systems., E hh p半导体光电材料基础Statistical Explanation of Wave Functions Probability Wave2*lWave function describes the quantum states of a microscopic particle.lProbability wave (Born, 1926):unifying the wavelike and particulate properties of microscopic particles. “Particulate”
19、 microscopic particles have certain masses and charges, but different from classic particles. “Wavelike” additivity of waves, but different from classic waves, which are the fluctuations of physical quantities in the space. is also called amplitude of probability wave.( , )r t( , )r tlProbability de
20、nsity , the probability of finding the particle in a unit volume near the position at time t. r半导体光电材料基础Schrdinger Equation The Schrdinger equation, a fundamental equation in quantum mechanics, provides the basic laws of matter movement in the microscopic world. Its actually a hypothesis, which need
21、s to be verified by experiments. ( )V r2222222xyz 22( , )( )( , )( , )2ir tV rr tHr ttm , : HamiltonianHFor a microscopic particle moving in a potential ,半导体光电材料基础Energy Eigenequation22( )( )( )2EEV rrErm If is independent of t, the solution can be obtained using the separation of variables where sa
22、tisfies the time-independent Schrdinger equation, /( , )( )iEtEr tr e( )Er In solving specific problems, the boundary conditions (bound states, periodic) requires that only certain values of E are acceptable, which are called the energy eigenvalues, and the corresponding solutions are called energy
23、eigenfunctions. Equation is the energy eigenequation of particles.( )Er( )V rE: energy of the system.( , )r t半导体光电材料基础 Energy Eigenfunction of the Hydrogen Atom Model201( )4eV rr Coulomb attraction: ( ,)r ( )rThe energy eigenequation of the electron in a hydrogen atom,22222222201112()(sin)()0sinsin4
24、erErrrrrr Only one electron outside the nucleus, so the energy eigenfunctions can be accurately solved!, reduced effective mass of electron.epepm mmmIn spherical coordinates:r r xyz+ +-( , , )( ) ( )( )rR r 半导体光电材料基础2222222222001(sin) (1)0sinsin12(1)()()04dmdddml lddddRel lrERr drdrrr : Periodic bou
25、ndary condition m =0,1,2,: Finite solution |m| l, l = 0,1,2,: For E 0, solutions always exist. (continuum-states) For E 0, bound-states boundary condition (r , R 0)(2 )( ) n=1,2,3,n-l-1=0,1,2,22202, 42( , , )( )( , )nnlmnllmeEEaeanrRr Y (Bohr radius)The energy levels E are quantized.半导体光电材料基础 Three
26、Quantum Numbers1. Principal quantum number n, n1, 2, 3,2. Orbital angular momentum quantum number l, l =0, 1, 2, (n 1)3. Magnetic quantum number m, m0, 1, 2,, llEach set of quantum numbers n, l, m determines a wave function of electron or an atomic orbital, and represent a state of motion of atomic
27、electron, different from the concept of Bohrs “atomic orbital”.lElectron cloud: describe the area around a nucleus where an electron will probably be, with density of dots representing the density of probability |2.半导体光电材料基础1. Principal quantum number nuDetermining the energy levels of hydrogen atom
28、. n are positive integers, and the energy levels are quantized.uA larger n means the electron is most likely to be found farther from the nucleus. The most probable radius,uAtomic orbitals with the same principal quantum number n are called one “electronic shell or shell”. The shells corresponding t
29、o n=1, 2, 3, 4, 5, 6, etc. are represented by labels K, L, M, N, O, P, etc.2, 1,2,3,.nrn an半导体光电材料基础2. Orbital angular momentum quantum number luDetermining the value of orbital angular momentum vector L of electrons, . uDescribing the shape of atomic orbitals and electron clouds. The orbitals with
30、l=0,1, 2,3, are represented by s,p,d,f.u Wave functions with the same l are called one “subshell”.uIn multi-electron atoms, l also determines the energy of electrons. ) 1( llL) 1( , 2 , 1 , 0nlQuantized orbital angular momentum半导体光电材料基础 3. Magnetic quantum number muThe orientation of orbital angular
31、 momentum vector is quantized. The projection of orbital angular momentum in z-axis is:uDescribing the orientation of atomic orbitals and electron clouds.zLmFor s-orbital, l=0, m=0. The electron cloud is spherical about the nucleus, no orientation.For p-orbital, l=1, m=0,1. The electron cloud has th
32、ree different orientations.For d-orbital, l=2, m=0,1,2. The electron cloud has five different orientations.m0,1,2,,l半导体光电材料基础1s: n=1, l=0, m=02p: n=2, l=1, m=1, 0 3d: n=3, l=2, m=2, 1, 0, 1s, 2p, 3d Electron Clouds of Hydrogen Atom半导体光电材料基础Spin Angular Momentum Quantum Number msuSpin is the intrinsi
33、c property of electrons. The spin angular momentum is, u The projection of electron spin in certain direction is,ms=1/2, the spin quantum number. uThe fourth quantum number, describing the electron state of motion (the spin orientation of the electron). uNot deriving from the Schrdinger equation, an
34、d not related to n, l, m. s=1/2) 1( ssLssszmL 半导体光电材料基础A Complete Description of the Motion States of Electrons Each set of n, l, m quantum numbers describes all the characteristics of one wave function, and thus determines the characteristics of the electron cloud. However, in order to completely d
35、escribe the motion states of atomic electrons, the spin quantum number ms is also need to be nailed down.半导体光电材料基础 Aufbau Principle (Lowest-energy principle) Electrons enter orbitals of lowest energy first. Pauli exclusion principle Within an orbital there can only be two electrons and each should h
36、ave opposite spins. (One spins clockwise and one spins counterclockwise.) Hunds rule When electrons occupy orbitals of the same energy, electrons will enter empty orbitals first, and with the same spins.Electron Configuration半导体光电材料基础2.1 Electron states in One Atom2.2 Electron States and Band Struct
37、ures in Semiconductors2.3 Impurity and Defect Energy Levels2.4 Carrier Distribution in a Semiconductor2.5 Conduction Processes in Semiconductors2.6 Non-equilibrium Charge Carriers2. Basics of Semiconductor Physics半导体光电材料基础2.2 Electron states and Band Structures in Semiconductorsu Electron Sharing Qu
38、alitative explanationu Band Theory Quantitative explanationu Interpretation of Metals, Semiconductors, and Insulators with Band Theoryu Quantum Confinement Effect半导体光电材料基础The periodicity in the structure of single crystals.Periodic potential Under single-electron approximation, the potential for eve
39、ry electron in the single crystals is regarded as periodic( )V r( )( )()mV rV rV rRmR arbitrary lattice vector.Electron Sharing and Formation of Band半导体光电材料基础V(r)rElectron Sharing and Formation of BandOne dimensional periodic potentialATOM半导体光电材料基础l Electron sharing Due to the overlap of electronic
40、shells between adjacent atoms that constitute a single crystal, electrons are not confined to a certain atom, and can transfer to the neighboring atom. So, electrons can move in the whole crystals, which is called electron sharing. The extent of overlap is larger for outer shells, therefore, the ele
41、ctron sharing is more prominent in the ourtermost shell, i.e. valence electrons.Electron Sharing and Formation of Band半导体光电材料基础l Electron sharing by Quantum Mechanics Based on the tunneling effect of quantum mechanics, electrons can cross the barrier between atoms and transfer to another atom, leadi
42、ng to the electron sharing in the whole crystal.Electron Sharing and Formation of Band半导体光电材料基础l Splitting of energy levels Due to the potentials of other atoms, the energy of shared electrons changes. The energy levels of isolated atoms will split.The closer of atoms, the stronger interaction betwe
43、en them, and the wider of band.Electron Sharing and Formation of BandH2 molecule半导体光电材料基础l Band structure General rules: The band related to the outer shell is wider. Smaller lattice constants lead to wider band. Bands can overlap.原子能级分裂为能带的示意图Electron Sharing and Formation of BandAllowed bandForbid
44、den bandAtomic levelAtomic orbitForbidden band半导体光电材料基础The number of energy levels in each band is N(2l+1). N the number of atoms, (2l+1) the degree of degeneracy of the atomic level of isolated atoms. E.g., If spin is ignored, since s-level is non-degenerate (m=0), when N atoms form a crystal, the
45、s-level splits into N close levels. While, the degree of degeneracy of p-level is 3 (m=0,1), so a p-level splits into 3N close energy levels. In practice, N1023, energy levels in each band get so close, that each band is considered as continuous, the so called “quasi-continuous”.Electron Sharing and
46、 Formation of Bandl Band structure半导体光电材料基础In practice, each band usually does not simply correspond to each energy level of isolated atoms. E.g., for Si and Ge, each atom has four valence electrons (2 s-electron, 2 p-electrons). When crystals form, due to orbital hybridization, the two bands do not
47、 correspond to s- and p-level, respectively, but both contains 2N levels. Each band could take in 4N electrons. Electron Sharing and Formation of Bandl Band structureUpper band: empty bandLower band: filled band半导体光电材料基础l Electron configuration in bandConfiguration rule: Lowest-energy principlePauli
48、 exclusion principlelBand related terms:Filled band:electron states are completely occupied by electrons. - Non-conductive Empty band: none of the states are occupied. - Non- conductive Partially-occupied band: parts of the states are occupied. - Conductive Electron Sharing and Formation of BandVale
49、nce band: the uppermost filled band of semiconductors, filled by valence electrons.Conduction band: the empty band above the valence band. 半导体光电材料基础lQuantitatively solving the electron states in single crystals by using quantum mechanics.The fundamental theory in current research of electron movemen
50、ts in solids.Clarifying the general rule of electron movements in single crystals.Demonstrating the differences among metals, semiconductors, and insulators.Band Theory半导体光电材料基础l Approximate theory Since a single crystal is consist of periodically arranged atoms, each of which contains many electron
51、s, the problem of electron movements in crystals is a many-body problem, which could not precisely solved.Band TheoryAdiabatic approximationSingle-electron approximationNearly free electron approximationTight binding approximation半导体光电材料基础l Adiabatic approximation Ignore the lattice vibration. Every
52、 ion core is fixed at its equilibrium position. (1927, Born-Oppenheimer)l Single-electron approximation Every electron is considered to move in an effective potential, and the movement of every electron is independent (Hartree-Fock) . For ideal single crystals, the effective potential is periodic.Ba
53、nd Theory半导体光电材料基础l Wave equation of electrons in crystal 22( )( )2V rrErm ( )()nV rV rR nR arbitrary lattice vector.Band Theorywhere半导体光电材料基础l Wave function of electrons in crystalBloch theorem if the potential is periodic, the solution of the wave equation should have the following form: ik rreu r
54、 where nu ru rR Bloch functionAnother expression: nik RnrRer nR arbitrary lattice vectorkwave vector( ) rBand Theory半导体光电材料基础l Bloch function ik rreu r nik RnrRer The electron wave functions for equivalent sites in different unit cells are differentiated by one factor with modulus of 1. The probabil
55、ity of finding electrons at equivalent sites in different unit cells is same.Band TheoryExpression 1:Expression 2:半导体光电材料基础l Wave function of free electrons2,i k rtr tAe For one freely moving electron with mass m, velocity v , its momentum p and energy E: de Broglie wave , for free electrons, plane
56、wave with frequency , and wavelength :2k wave vectork21, 2ppmvEm Band Theory, Ehph半导体光电材料基础( )ik rrAe 22|( )|rA - The probability of finding the electron at different places are the same - freely moving.Ehpk2hEkWave vector describes the electron states.k212pmvpEm 222kvmkEmBand Theoryl Wave function
57、of free electrons半导体光电材料基础l Periodic boundary condition and the value of For a finite crystal in a cuboid shape, which has N1, N2, N3 unit cells along directions of , , , so the Bloch function should satisfied the following periodic boundary condition:()( )kiikrN ar ik rreu r 1a2a 3a k(1,2,3)ia i la
58、ttice primitive vectorBand Theory半导体光电材料基础 Due to the periodic boundary condition, the wave vector should take some discrete values. ik rreu r 312123123lllkbbbNNN li integers,(1,2,3)ib i reciprocal lattice primitive vector.Wave vector represents the motion state of electrons in crystals. is not the
59、momentum any more. kkBand Theoryl Periodic boundary condition and the value of kk半导体光电材料基础 ik rreu r lGeneral conclusions for electron motion states in crystals. The states of Bloch electrons are decided by the two quantum numbers n and k , and the corresponding energy eigenvalue and wave function a
60、re and For given n, is a periodic function of , which has upper and lower limits. Different n represents different bands. Constitutes the band structure of crystals.( )nEk( )nkr( )nEkk( )nEkBand Theory半导体光电材料基础( )nEkkas a function ofEnergy BandReduced Brillouin Zonek k22Band TheoryBrillouin zone1st2nd2nd3r
温馨提示
- 1. 本站所有资源如无特殊说明,都需要本地电脑安装OFFICE2007和PDF阅读器。图纸软件为CAD,CAXA,PROE,UG,SolidWorks等.压缩文件请下载最新的WinRAR软件解压。
- 2. 本站的文档不包含任何第三方提供的附件图纸等,如果需要附件,请联系上传者。文件的所有权益归上传用户所有。
- 3. 本站RAR压缩包中若带图纸,网页内容里面会有图纸预览,若没有图纸预览就没有图纸。
- 4. 未经权益所有人同意不得将文件中的内容挪作商业或盈利用途。
- 5. 人人文库网仅提供信息存储空间,仅对用户上传内容的表现方式做保护处理,对用户上传分享的文档内容本身不做任何修改或编辑,并不能对任何下载内容负责。
- 6. 下载文件中如有侵权或不适当内容,请与我们联系,我们立即纠正。
- 7. 本站不保证下载资源的准确性、安全性和完整性, 同时也不承担用户因使用这些下载资源对自己和他人造成任何形式的伤害或损失。
最新文档
- 2026年保定职业技术学院单招职业倾向性测试题库附答案详解(综合题)
- 2026年佳木斯职业学院单招综合素质考试题库及答案详解(历年真题)
- 2026年南京铁道职业技术学院单招职业适应性测试题库及答案详解(名校卷)
- 2026年北海职业学院单招职业倾向性测试题库带答案详解(培优)
- 2026年佳木斯职业学院单招职业倾向性测试题库带答案详解(典型题)
- 2026年信阳航空职业学院单招职业倾向性考试题库参考答案详解
- 2026年南充电影工业职业学院单招职业倾向性考试题库附参考答案详解(考试直接用)
- 2026年内蒙古能源职业学院单招综合素质考试题库附参考答案详解(基础题)
- 2026年内蒙古呼伦贝尔市单招职业倾向性测试题库附参考答案详解(突破训练)
- 托育师QC管理能力考核试卷含答案
- 东北三省三校哈尔滨师大附中2026届高三毕业班质量检测试题(A)数学试题试卷含解析
- 江苏苏州工业园区2025-2026学年九年级第一学期历史期末调研试卷(试卷+解析)
- 八下语文必读名著《经典常谈》考点梳理
- 2026年七年级数学春季开学第一课
- 集装箱焊接制度规范要求
- 第五范式-人工智能驱动的科技创新
- 高标准农田建设工程质量专项整治技术手册(2025年版)
- DB4406∕T 53-2025 老年人陪诊服务规范
- 2026豫信电子科技集团招聘面试题及答案
- 校园轻食店创业计划书
- 污水处理站调度与维护施工方案

评论
0/150
提交评论