



版权说明:本文档由用户提供并上传,收益归属内容提供方,若内容存在侵权,请进行举报或认领
文档简介
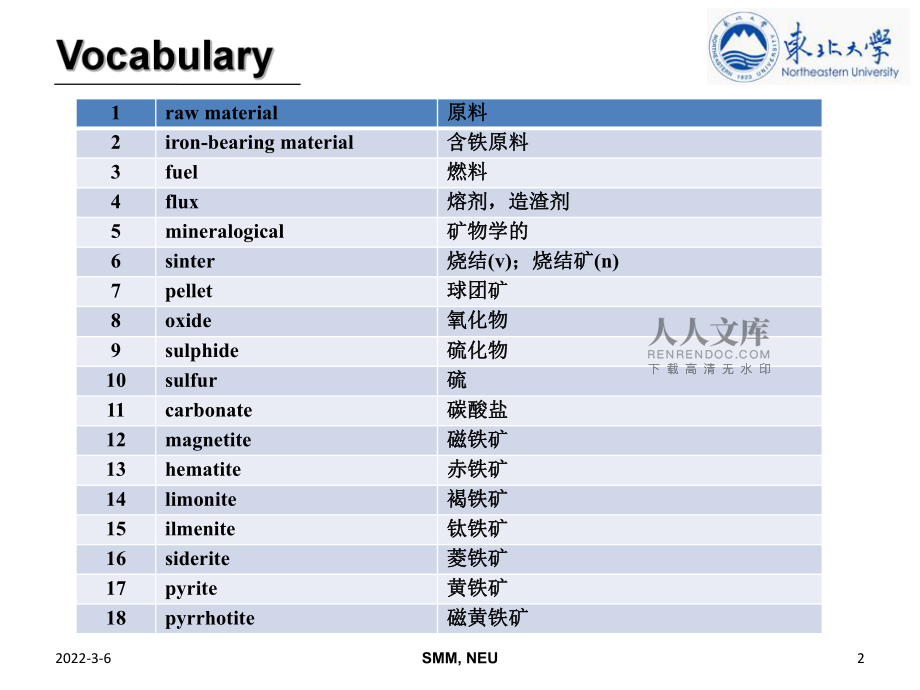
1、SMM, NEU2022-3-621raw material原料原料2iron-bearing material含铁原料含铁原料3fuel燃料燃料4flux熔剂,造渣剂熔剂,造渣剂5mineralogical矿物学的矿物学的6sinter烧结烧结(v);烧结矿;烧结矿(n)7pellet球团矿球团矿8oxide氧化物氧化物9sulphide硫化物硫化物10sulfur硫硫11carbonate碳酸盐碳酸盐12magnetite磁铁矿磁铁矿13hematite赤铁矿赤铁矿14limonite褐铁矿褐铁矿15ilmenite钛铁矿钛铁矿16siderite菱铁矿菱铁矿17pyrite黄铁矿黄铁矿1
2、8pyrrhotite磁黄铁矿磁黄铁矿SMM, NEU2022-3-6319ferrous含铁的,亚铁的含铁的,亚铁的20ferric铁的,三价铁的铁的,三价铁的21gangue脉石脉石22titanium钛钛23hydrous含水的含水的24reducible可还原的可还原的25rock岩石岩石26crystallization结晶化结晶化27water of crystallization结晶水结晶水28alumina氧化铝氧化铝29charge炉料炉料(n);装炉,装料;装炉,装料(v)30agglomerate结块,烧结结块,烧结31pelletize使成颗粒状使成颗粒状32grate
3、箅条,固定筛箅条,固定筛33ignite着火,点火着火,点火34frit熔融熔融(化化)35particle粒子,极小量粒子,极小量36pulverize使使成粉末成粉末SMM, NEU2022-3-6437pulverized粉状的粉状的38ultrafine极其细小的极其细小的39concentrate精矿,精煤精矿,精煤40binder粘合剂粘合剂41green pellet生球生球42shaft-type furnace竖炉竖炉43kiln窑窑44rotary kiln回转窑回转窑45permeability渗透,渗透性渗透,渗透性46storage储藏储藏47swell膨胀膨胀48s
4、tick粘住,粘贴粘住,粘贴49metallic金属的,含金属的金属的,含金属的50carburize使渗碳使渗碳51strength强度强度52structural结构结构(上上)的的53soft coal烟煤烟煤54hard coal无烟煤无烟煤SMM, NEU2022-3-6555injection喷吹,喷射喷吹,喷射56pulverized coal injection喷煤喷煤57dramatically鲜明地,显著地鲜明地,显著地58coke rate焦比焦比59approach方法,途径方法,途径60advance进步,进展进步,进展61limestone石灰石石灰石62ash灰分
5、灰分63drain排出,流掉排出,流掉64sulphur holding power脱硫能力脱硫能力65fluidity流动性流动性66portion部分,一份部分,一份67dependency依靠,依赖依靠,依赖68harden使变硬,使坚强使变硬,使坚强69traveling grate移动床移动床70compound化合物化合物71chemical composition化学成分化学成分72reddish淡红色的,微红的淡红色的,微红的SMM, NEU2022-3-66 The raw materials for the production of iron in the blast f
6、urnace can be grouped as follows: Iron-bearing materials, fuels and fluxes. The major iron-bearing materials are iron ores, sinter and pellet in the blast furnace. Their function is to supply the element iron, which is 93 to 94 percent of the pig iron produce. Iron ores are classed by their chemical
7、 compositions, such as oxides, sulfides, carbonates, etc, as shown in the table below.Chemical composition of pure mineralCommon designationMagnetiteFe3O4Ferrous-ferric oxideHematiteFe2O3Ferric oxideIlmeniteFeTiO3Iron-titanium oxideLimonitenFe2O3mH2OHydrous iron oxideSideriteFeCO3Iron carbonatePyrit
8、e (iron pyrites)FeS2Pyrrhotite (magnetic iron pyrites)FeSIron sulphidesSMM, NEU2022-3-67 Hematite is one of the most widely used ores. If pure, it would give 70% iron. The typical reddish color is caused by the iron (III). In the case of red iron ore, the compound of iron and oxygen is not so “tight
9、” that the hematite is regarded as “easily reducible”. Magnetite, a magnetic iron ore, is increasing in use for two reasons. Firstly, it can be separated from the rock by magnetic means; secondly, it has high iron content. Iron and oxygen atoms are very closely combined with each other in magnetite,
10、 thus making magnetite “difficult to reduce”. Limonite is a brown iron ore and contains water, which means that the iron oxides have formed a stable compound with water (water of crystallization). Containing 30 to 40% Fe, siderites are relatively easy to reduce. Most ores contain only 50 to 60% iron
11、 because they contain 10 to 20% gangue (which consists mostly of alumina and silica). If the gangue contains mainly lime, the ore is “basic”; if silicon oxide (SiO2) predominates, the ore is “acid”.SMM, NEU2022-3-68 The portion of the ore that is too fine to be charged directly into blast furnace is
12、 usually agglomerated. The most important processes are: sintering and pelletizing. The sintering process is in five stages: (1) mixing of the raw materials and fine coal/or coke, (2) placing the mixture on a grate, (3) igniting and sintering. Air drawn through the mixture burns the fuel at a temper
13、ature high enough to frit the small particles together into a cake so that they can be charged into the blast furnace satisfactorily, (4) cooling, (5) crushing and screening before charging to the furnaces. For best results, pulverized flux is added to the sinter mix to combine with the gangue of th
14、e ore in the sintering process. Sinter usually contains 50 to 60% iron. During pelletizing, the mixtures made from ultrafine (minus 0.074mm or minus 200 mesh) iron ore concentrates and binders of grain sizes far less than 1mm are balled to form “green” pellets slightly larger than 6mm but smaller th
15、an 15mm in diameter. The green pellets SMM, NEU2022-3-69are then hardened by firing in a shaft-type furnace or rotary kiln or on a traveling grate. Pellets usually contain from 60 to 67% of iron. Compared with lump ores and sinter, the advantages of pellets are: a narrow size range, constant quality
16、 and good permeability during reduction. Furthermore, pellets are well suited for transport and storage. But, any swelling and sticking of pellets during the reduction phase must be avoided. The fuels enter the blast furnace as coke, coal, oil or gas. They are used for producing the heat required fo
17、r smelting, and reducing the iron oxides into metallic iron and carburizing the iron (about 40 to 50 kilograms per ton of iron). In addition, because the coke retains its strength at high temperature, it provides the structural support that keeps the unmelted burden materials from falling into the h
18、earth. SMM, NEU2022-3-610 At present, some of the coke in the blast furnace is usually replaced by coal. The blast furnace can inject hard coal, soft coal and mixed coal. BF pulverized coal injection can dramatically reduce coke rate and the dependency on increasing shortage of coke resource, so it
19、is the most effective approach to reducing the ironmaking cost and has become an important part in BF ironmaking technology advances. Fluxes include limestone, dolomite and lime mainly, whose major functions are to combine with the ash in the coke and the acid gangue in the ores to make a fluid slag
20、 that can be drained readily from the furnace hearth. The ratio of basic oxides to acid oxides must be controlled carefully to preserve the sulphur-holding power of the slag as the fluidity.SMM, NEU2022-3-611What are raw materials for the production of iron at the BF?Why is magnetite increasing in u
21、se of iron making?Can you describe the sintering process?What are functions of the coke?What coals can be injected in the blast furnace?Why must the ratio of basic oxides to acid oxides be controlled carefully?What are the advantages of pellets compared with lump ores?What is some of the coke in the
22、 blast furnace usually replaced by at present?What do fluxes mainly include?SMM, NEU2022-3-612 1. 炼铁原料炼铁原料 2. 易还原矿石易还原矿石 3. 酸性脉石酸性脉石 4. 碱性脉石碱性脉石 5. 球团矿球团矿 6. 含铁原料含铁原料 7. 喷煤喷煤 8. 磁铁矿磁铁矿 9. 赤铁矿赤铁矿 10. 焦炭灰分焦炭灰分 11. 烧结矿烧结矿 12. 熔剂熔剂SMM, NEU2022-3-6131. The major iron b_ materials are iron ores, sinter an
23、d pellets.2. Iron oxides are always mixed with impurities which are called the g_.3. If the gangue contains mainly lime, the ore is b_.4. H_ is one of the most widely used ores.5. L_ is a brown iron ore.6. The portion of the ore that is too fine to be charged directly is usually a_.7. Compared with
24、lump ores, p_ have a narrow size range, constant quality and good permeability during reduction.8. The f_ enter the blast furnace as coke, coal, oil or gas.9. Fluxes include l_, d_ and lime mainly.SMM, NEU2022-3-614Hematite is _ (one; a; an) of the most widely used ores. If pure, would give 70% iron
25、. The typical reddish color is caused by the iron (III). In the case of red iron ore, the compound of iron and oxygen _ (are; am; is) not so “tight” and so the _ (magnetite; hematite) is regarded as “easily reducible”.Iron and oxygen atoms are very closely _ (combined; combining; combines) with each
26、 other in magnetite, thus making magnetite “difficult to reduce”. Limonite _ (is; are; was) an brown iron ore. It contains water, which means that the iron oxides have formed a stable compound with water (water of crystallization).SMM, NEU2022-3-6151. Iron ores are classed by their chemical compositions, such as oxides, carbonates
温馨提示
- 1. 本站所有资源如无特殊说明,都需要本地电脑安装OFFICE2007和PDF阅读器。图纸软件为CAD,CAXA,PROE,UG,SolidWorks等.压缩文件请下载最新的WinRAR软件解压。
- 2. 本站的文档不包含任何第三方提供的附件图纸等,如果需要附件,请联系上传者。文件的所有权益归上传用户所有。
- 3. 本站RAR压缩包中若带图纸,网页内容里面会有图纸预览,若没有图纸预览就没有图纸。
- 4. 未经权益所有人同意不得将文件中的内容挪作商业或盈利用途。
- 5. 人人文库网仅提供信息存储空间,仅对用户上传内容的表现方式做保护处理,对用户上传分享的文档内容本身不做任何修改或编辑,并不能对任何下载内容负责。
- 6. 下载文件中如有侵权或不适当内容,请与我们联系,我们立即纠正。
- 7. 本站不保证下载资源的准确性、安全性和完整性, 同时也不承担用户因使用这些下载资源对自己和他人造成任何形式的伤害或损失。
最新文档
- 重庆2025年重庆市属事业单第三季度招聘更正笔试历年参考题库附带答案详解
- 许昌2025年河南许昌职业技术学院招聘13人笔试历年参考题库附带答案详解
- 舟山浙江舟山东港街道招聘后勤工作人员(一)笔试历年参考题库附带答案详解
- 白银2025年甘肃白银市精神卫生中心招聘护理人员笔试历年参考题库附带答案详解
- 职业人群颈椎病的精准干预方案
- 桂林2025年广西桂林市七星区基层医疗卫生事业单位招聘专业技术人员笔试历年参考题库附带答案详解
- 无锡2025年江苏无锡宜兴市人民法院招聘编外用工人员6人笔试历年参考题库附带答案详解
- 德州2025年山东德州乐陵市审计局引进急需紧缺人才2人笔试历年参考题库附带答案详解
- 崇左2025年广西崇左市龙州县卫生健康事业单位招聘107人笔试历年参考题库附带答案详解
- 安庆2025年安徽安庆大观经济开发区招聘工作人员笔试历年参考题库附带答案详解
- T∕CAMH 00002-2025 心理咨询师职业能力水平评价标准
- 2025年小学蔬菜颁奖典礼
- DB4114∕T 250-2024 农民田间学校建设管理规范
- 急诊科胸部创伤救治指南
- 二手手机计划书项目方案
- 十年(2016-2025年)高考数学真题分类汇编:专题10 数列解答题综合一(原卷版)
- 医院保洁人员安全管理与保障制度
- 工业园区规划(环境影响评价、水资源论证、安全风险评估等)方案咨询服务投标文件(技术标)
- 2024低温低浊水给水处理设计标准
- 《房屋市政工程生产安全重大事故隐患判定标准(2024版)》解读
- 2025年国资委公务员面试热点问题集锦及答案

评论
0/150
提交评论