



版权说明:本文档由用户提供并上传,收益归属内容提供方,若内容存在侵权,请进行举报或认领
文档简介
OrganicChemistryWilliamH.BrownChristopherS.FooteBrentL.IversonOrganicChemistryWilliamH.BrCovalentBonding&ShapesofMoleculesChapter1
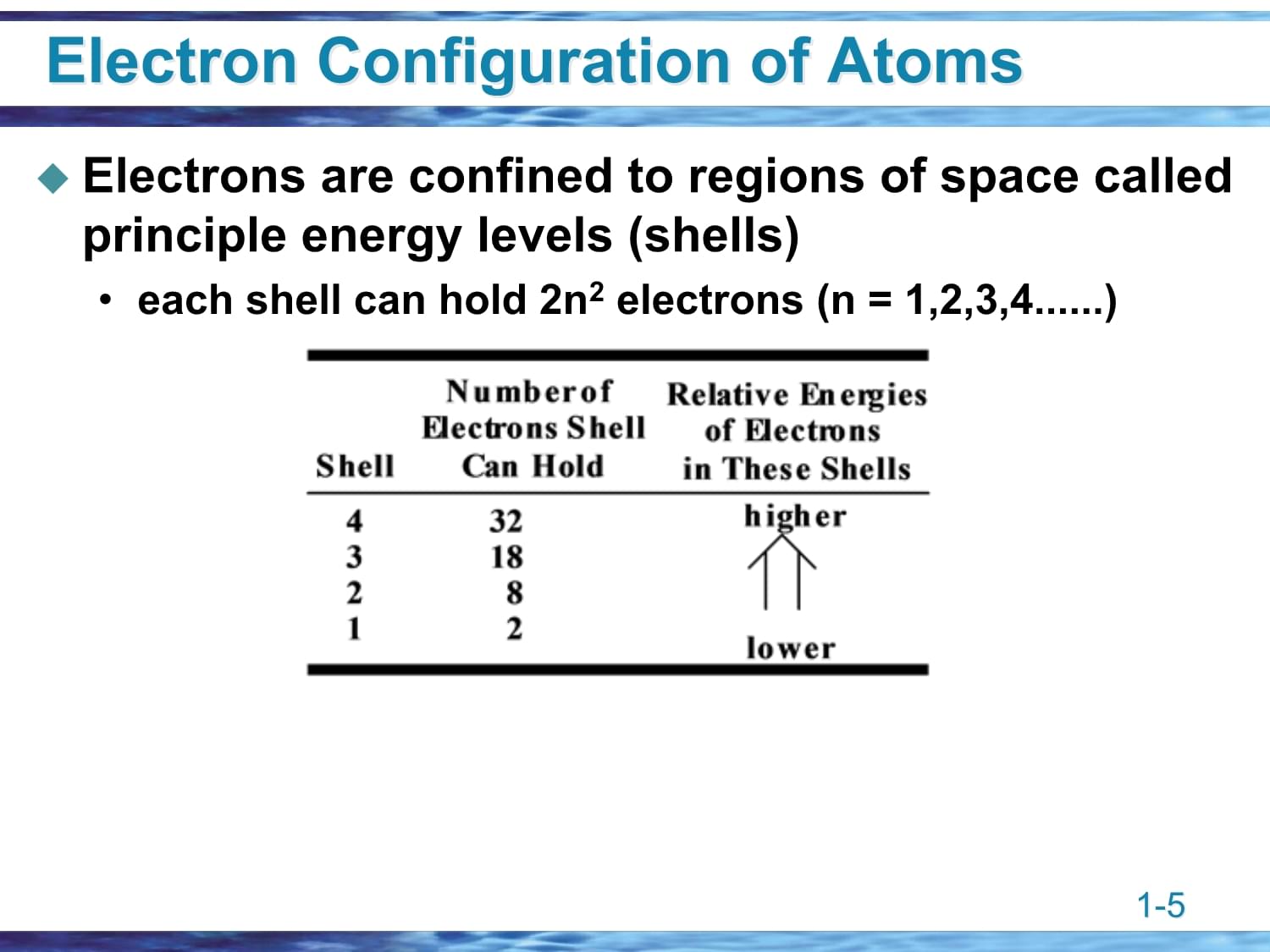
CovalentBonding&ShapesofMOrganicChemistryThestudyofthecompoundsofcarbonOver10millioncompoundshavebeenidentifiedabout1000newonesareidentifiedeachday!Cisasmallatomitformssingle,double,andtriplebondsitisintermediateinelectronegativity(2.5)itformsstrongbondswithC,H,O,N,andsomemetalsOrganicChemistryThestudyofSchematicViewofanAtomasmalldensenucleus,diameter10-14-10-15m,whichcontainspositivelychargedprotonsandmostofthemassoftheatomanextranuclearspace,diameter10-10m,whichcontainsnegativelychargedelectronsSchematicViewofanAtomasmaElectronConfigurationofAtomsElectronsareconfinedtoregionsofspacecalledprincipleenergylevels(shells)eachshellcanhold2n2electrons(n=1,2,3,4......)ElectronConfigurationofAtomElectronConfigurationofAtomsShellsaredividedintosubshellscalledorbitals,whicharedesignatedbytheletterss,p,d,f,........s(onepershell)p(setofthreepershell2andhigher)d(setoffivepershell3andhigher).....ElectronConfigurationofAtomElectronConfigurationofAtomsAufbauPrinciple:
orbitalsfillinorderofincreasingenergyfromlowestenergytohighestenergyPauliExclusionPrinciple:
onlytwoelectronscanoccupyanorbitalandtheirspinsmustbepairedHund’sRule:
whenorbitalsofequalenergyareavailablebuttherearenotenoughelectronstofillallofthem,oneelectronisaddedtoeachorbitalbeforeasecondelectronisaddedtoanyoneofthemElectronConfigurationofAtomElectronConfigurationofAtomsThepairingofelectronspinsElectronConfigurationofAtomElectronConfigurationofAtomsTable1.3TheGround-StateElectronConfigurationofElements1-18ElectronConfigurationofAtomLewisDotStructuresGilbertN.LewisValenceshell:
theoutermostoccupiedelectronshellofanatomValenceelectrons:
electronsinthevalenceshellofanatom;theseelectronsareusedtoformchemicalbondsandinchemicalreactionsLewisdotstructure:
thesymbolofanelementrepresentsthenucleusandallinnershellelectronsdotsrepresentvalenceelectronsLewisDotStructuresGilbertN.LewisDotStructuresTable1.4LewisDotStructuresforElements1-18LewisDotStructuresTable1.4LewisModelofBondingAtomsbondtogethersothateachatomacquiresanelectronconfigurationthesameasthatofthenoblegasnearestitinatomicnumberanatomthatgainselectronsbecomesananionanatomthatloseselectronsbecomesacationtheattractionofanionsandcationsleadstotheformationofionicsolidsanatommayshareelectronswithoneormoreatomstocompleteitsvalenceshell;achemicalbondformedbysharingelectronsiscalledacovalentbondbondsmaybepartiallyionicorpartiallycovalent;thesebondsarecalledpolarcovalentbondsLewisModelofBondingAtomsboElectronegativityElectronegativity:
ameasureofanatom’sattractionfortheelectronsitshareswithanotheratominachemicalbondPaulingscalegenerallyincreaseslefttorightinarowgenerallyincreasesbottomtotopinacolumnElectronegativityElectronegatiFormationofIonsAroughguideline:ionswillformifthedifferenceinelectronegativitybetweeninteractingatomsis1.9orgreaterexample:sodium(EN0.9)andfluorine(EN4.0)weuseasingle-headed(barbed)curvedarrowtoshowthetransferofoneelectronfromNatoFinformingNa+F-,thesingle3selectronfromNaistransferredtothepartiallyfilledvalenceshellofFFormationofIonsAroughguideCovalentBondsThesimplestcovalentbondisthatinH2thesingleelectronsfromeachatomcombinetoformanelectronpairthesharedpairfunctionsintwowayssimultaneously;itissharedbythetwoatomsandfillsthevalenceshellofeachatomThenumberofsharedpairsonesharedpairformsasinglebondtwosharedpairsformadoublebondthreesharedpairsformatriplebondCovalentBondsThesimplestcovPolarandNonpolarCovalentBondsAlthoughallcovalentbondsinvolvesharingofelectrons,theydifferwidelyinthedegreeofsharingWedividecovalentbondsintononpolarcovalentbondspolarcovalentbondsPolarandNonpolarCovalentBoPolarandNonpolarCovalentBondsanexampleofapolarcovalentbondisthatofH-ClthedifferenceinelectronegativitybetweenClandHis3.0-2.1=0.9weshowpolaritybyusingthesymbolsd+andd-,orbyusinganarrowwiththearrowheadpointingtowardthenegativeendandaplussignonthetailofthearrowatthepositiveendPolarandNonpolarCovalentBoPolarCovalentBondsBonddipolemoment(m):
ameasureofthepolarityofacovalentbondtheproductofthechargeoneitheratomofapolarbondtimesthedistancebetweenthenucleiTable1.7showsaveragebonddipolemomentsofselectedcovalentbondsPolarCovalentBondsBonddipolLewisStructuresTowriteaLewisstructuredeterminethenumberofvalenceelectronsdeterminethearrangementofatomsconnecttheatomsbysinglebondsarrangetheremainingelectronssothateachatomhasacompletevalenceshellshowabondingpairofelectronsasasinglelineshowanonbondingpairofelectronsasapairofdotsinasinglebondatomsshareonepairofelectrons,inadoublebondtheysharetwopairsofelectrons,andinatriplebondtheysharethreepairsofelectronsLewisStructuresTowriteaLewLewisStructures-Table1.3Inneutralmoleculeshydrogenhasonebondcarbonhas4bondsandnolonepairsnitrogenhas3bondsand1lonepairoxygenhas2bondsand2lonepairshalogenshave1bondand3lonepairsLewisStructures-Table1.3FormalChargeFormalcharge:thechargeonanatominamoleculeorapolyatomicionToderiveformalcharge1.writeacorrectLewisstructureforthemoleculeorion2.assigneachatomallitsunshared(nonbonding)electronsandone-halfitsshared(bonding)parethisnumberwiththenumberofvalenceelectronsintheneutral,unbondedatomFormalChargeFormalcharge:thFormalChargeExample:
DrawLewisstructures,andshowwhichatomineachbearstheformalchargeFormalChargeExample:DrawLewExceptionstotheOctetRuleMoleculescontainingatomsofGroup3Aelements,particularlyboronandaluminumAluminumchloride:::FBFFClAlClCl6electronsinthevalenceshellsofboronandaluminumBorontrifluoride:::::::::::::::ExceptionstotheOctetRuleMoExceptionstotheOctetRuleAtomsofthird-periodelementshave3dorbitalsandmayexpandtheirvalenceshellstocontainmorethan8electronsphosphorusmayhaveupto10ExceptionstotheOctetRuleAtExceptionstotheOctetRulesulfur,anotherthird-periodelement,formscompoundsinwhichitsvalenceshellcontains8,10,or12electronsExceptionstotheOctetRulesuFunctionalGroupsFunctionalgroup:anatomorgroupofatomswithinamoleculethatshowsacharacteristicsetofphysicalandchemicalpropertiesFunctionalgroupsareimportantforthreereason;theyare1.theunitsbywhichwedivideorganiccompoundsintoclasses2.thesitesofcharacteristicchemicalreactions3.thebasisfornamingorganiccompoundsFunctionalGroupsFunctionalgrAlcoholscontainan-OH(hydroxyl)groupEthanolmayalsobewrittenasacondensedstructuralformulaAlcoholscontainan-OH(hydroxAlcoholsalcoholsareclassifiedasprimary(1°),secondary(2°),ortertiary(3°)dependingonthenumberofcarbonatomsbondedtothecarbonbearingthe-OHgroupAlcoholsalcoholsareclassifieAlcoholstherearetwoalcoholswithmolecularformulaC3H8OAlcoholstherearetwoalcoholsAminescontainanaminogroup;ansp3-hybridizednitrogenbondedtoone,two,orthreecarbonatomsanaminemayby1°,2°,or3°CH3NHHCH3NHCH3CH3NCH3CH3Methylamine(a1°
amine)Dimethylamine(a2°
amine)Trimethylamine(a3°
amine):::Aminescontainanaminogroup;AldehydesandKetonescontainacarbonyl(C=O)groupAldehydesandKetonescontainaCarboxylicAcidscontainacarboxyl(-COOH)groupCarboxylicAcidscontainacarbCarboxylicEstersEster:aderivativeofacarboxylicacidinwhichthecarboxylhydrogenisreplacedbyacarbongroupCarboxylicEstersEster:aderiCarboxylicAmideCarboxylicamide,commonlyreferredtoasanamide:aderivativeofacarboxylicacidinwhichthe-OHofthe-COOHgroupisreplacedbyanaminethesixatomsoftheamidefunctionalgrouplieinaplanewithbondanglesofapproximately120°CarboxylicAmideCarboxylicamiVSEPRBasedonthetwinconceptsthatatomsaresurroundedbyregionsofelectrondensityregionsofelectrondensityrepeleachotherVSEPRBasedonthetwinconceptVSEPRModelExample:
predictallbondanglesforthesemoleculesandionsVSEPRModelExample:predictalPolarandNonpolarMoleculesTodetermineifamoleculeispolar,weneedtodetermineifthemoleculehaspolarbondsthearrangementofthesebondsinspaceMoleculardipolemoment():thevectorsumoftheindividualbonddipolemomentsinamoleculereportedindebyes(D)PolarandNonpolarMoleculesToPolarandNonpolarMoleculesthesemoleculeshavepolarbonds,buteachhasazerodipolemomentPolarandNonpolarMoleculesthPolarandNonpolarMoleculesthesemoleculeshavepolarbondsandarepolarmoleculesPolarandNonpolarMoleculesthPolarandNonpolarMoleculesformaldehydehaspolarbondsandisapolarmoleculePolarandNonpolarMoleculesfoResonanceFormanymoleculesandions,nosingleLewisstructureprovidesatrulyaccuraterepresentationResonanceFormanymoleculesanResonanceLinusPauling-1930smanymoleculesandionsarebestdescribedbywritingtwoormoreLewisstructuresindividualLewisstructuresarecalledcontributingstructuresconnectindividualcontributingstructuresbydouble-headed(resonance)arrowsthemoleculeorionisahybridofthevariouscontributingstructuresResonanceLinusPauling-1930sResonanceExamples:
equivalentcontributingstructuresResonanceExamples:equivalentResonanceCurvedarrow:
asymbolusedtoshowtheredistributionofvalenceelectronsInusingcurvedarrows,thereareonlytwoallowedtypesofelectronredistribution:fromabondtoanadjacentatomfromanatomtoanadjacentbondElectronpushingisasurvivalskillinorganicchemistrylearnitwell!ResonanceCurvedarrow:asymboResonanceAllcontributingstructuresmust1.havethesamenumberofvalenceelectrons2.obeytherulesofcovalentbondingnomorethan2electronsinthevalenceshellofHnomorethan8electronsinthevalenceshellofa2ndperiodelement3rdperiodelements,suchasPandS,mayhaveupto12electronsintheirvalenceshells3.differonlyindistributionofvalenceelectrons;thepositionofallnucleimustbethesame4.havethesamenumberofpairedandunpairedelectronsResonanceAllcontributingstruResonanceThecarbonateion,forexampleahybridofthreeequivalentcontributingstructuresthenegativechargeisdistributedequallyamongthethreeoxygensResonanceThecarbonateion,foResonancePreference1:filledvalenceshellsstructuresinwhichallatomshavefilledvalenceshellscontributemorethanthosewithoneormoreunfilledvalenceshells••••••Greatercontribution;bothcarbonandoxygenhavecompletevalenceshellsLessercontribution;carbonhasonly6electronsinitsvalenceshell++CCH3OCH3OHHCHHResonancePreference1:filledResonancePreference2:maximumnumberofcovalentbondsstructureswithagreaternumberofcovalentbondscontributemorethanthosewithfewercovalentbondsResonancePreference2:maximumResonancePreference3:leastseparationofunlikechargestructureswithseparationofunlikechargescontributelessthanthosewithnochargeseparationLessercontribution(separationofunlikecharges)CH3-C-CH3CH3-C-CH3Greatercontribution(noseparationofunlikecharges)O
-O:::::ResonancePreference3:leastsResonancePreference4:negativechargeonthemoreelectronegativeatomstructuresthatcarryanegativechargeonthemoreelectronegativeatomcontributemorethanthosewiththenegativechargeonthelesselectronegativeatomResonancePreference4:negativQuantumorWaveMechanicsAlbertEinstein:E=hn(energyisquantized)lighthasparticlepropertiesLouisdeBroglie:wave/particledualityErwinSchrödinger:waveequationwavefunction,:asolutiontoasetofequationsthatdepictstheenergyofanelectroninanatomeachwavefunctionisassociatedwithauniquesetofquantumnumberseachwavefunctionoccupiesthree-dimensionalspaceandiscalledanorbital
2istheprobabilityoffindinganelectronatagivenpointinspaceQuantumorWaveMechanicsAlberShapesof1sand2sOrbitalsProbabilitydistribution(2)for1sand2sorbitalsshowinganarbitraryboundarysurfacecontainingabout95%oftheelectrondensityShapesof1sand2sOrbitalsPrShapesofaSetof2pAtomicOrbitalsThree-dimensionalshapesof2patomicorbitalsShapesofaSetof2pAtomicOMolecularOrbitalTheoryElectronsinatomsexistinatomicorbitalsElectronsinmoleculesexistinmolecularorbitals(MOs)UsingtheSchrödingerequation,wecancalculatetheshapesandenergiesofMOsMolecularOrbitalTheoryElectrMolecularOrbitalTheoryRules:combinationofnatomicorbitals(mathematicallyaddingandsubtractingwavefunctions)givesnMOs(newwavefunctions)MOsarearrangedinorderofincreasingenergyMOfillingisgovernedbythesamerulesasforatomicorbitals:Aufbauprinciple:fillbeginningwithLUMOPauliexclusionprinciple:nomorethan2e-inaMOHund’srule:whentwoormoreMOsofequivalentenergyareavailable,add1e-toeachbeforefillinganyoneofthemwith2e-MolecularOrbitalTheoryRules:MolecularOrbitalTheoryTerminologygroundstate=lowestenergystateexcitedstate=NOTlowestenergystate
=sigmabondingMO
*=sigmaantibondingMO
=pibondingMO
*=piantibondingMOHOMO=highestoccupiedMOLUMO=lowestunoccupiedMOMolecularOrbitalTheoryTerminMolecularOrbitalTheorySigma1sbondingandantibondingMOsMolecularOrbitalTheorySigmaMolecularOrbitalTheoryMOenergydiagramforH2:(a)groundstateand(b)lowestexcitedstateMolecularOrbitalTheoryMOeneMolecularOrbitalscomputedsigmabondingandantibondingMOsforH2MolecularOrbitalscomputedsigMolecularOrbitalspibondingandantibondingMOsMolecularOrbitalspibondingaMolecularOrbitalscomputedpibondingandantibondingMOsforethyleneMolecularOrbitalscomputedpiMolecularOrbitalscomputedpibondingandantibondingorbitalsforformaldehydeMolecularOrbitalscomputedpiHybridOrbitalsTheProblem:bondingby2sand2patomicorbitalswouldgivebondanglesofapproximately90°insteadweobservebondanglesofapproximately109.5°,120°,and180°ASolutionhybridizationofatomicorbitals2ndrowelementsusesp3,sp2,andsphybridorbitalsforbondingHybridOrbitalsTheProblem:HybridOrbitalsHybridizationoforbitals(L.Pauling)thecombinationoftwoormoreatomicorbitalsformsanewsetofatomicorbitals,calledhybridorbitalsWedealwiththreetypesofhybridorbitalssp3
(onesorbital+threeporbitals)sp2
(onesorbital+twoporbitals)sp(onesorbital+oneporbital)Overlapofhybridorbitalscanformtwotypesofbondsdependingonthegeometryofoverlapbonds
areformedby“direct”overlapbonds
areformedby“parallel”overlapHybridOrbitalsHybridizationosp3HybridOrbitalseachsp3hybridorbitalhastwolobesofunequalsizethesignofthewavefunctionispositiveinonelobe,negativeintheother,andzeroatthenucleusthefoursp3hybridorbitalsaredirectedtowardthecornersofaregulartetrahedronatanglesof109.5°sp3HybridOrbitalseachsp3hysp3HybridOrbitalsorbitaloverlappicturesofmethane,ammonia,andwatersp3HybridOrbitalsorbitalovesp2HybridOrbitalstheaxesofthethreesp2hybridorbitalslieinaplaneandaredirectedtowardthecornersofanequilateraltriangletheunhybridized2porbitalliesperpendiculartotheplaneofthethreehybridorbitalssp2HybridOrbitalstheaxesofBondinginEthyleneBondinginEthyleneBondinginFormaldehydeBondinginFormaldehydespHybridOrbitalstwolobesofunequalsizeatanangleof180°theunhybridized2porbitalsareperpendiculartoeachotherandtothelinecreatedbytheaxesofthetwosphybridorbitalsspHybridOrbitalstwolobesofBondinginAcetylene,C2H2BondinginAcetylene,C2H2HybridOrbitalsHybridOrbitalsBondLengthsandBondStrengthsBondLengthsandBondStrengthCovalentBonds&ShapesofMoleculesEndChapter1CovalentBonds&ShapesofMolOrganicChemistryWilliamH.BrownChristopherS.FooteBrentL.IversonOrganicChemistryWilliamH.BrCovalentBonding&ShapesofMoleculesChapter1
CovalentBonding&ShapesofMOrganicChemistryThestudyofthecompoundsofcarbonOver10millioncompoundshavebeenidentifiedabout1000newonesareidentifiedeachday!Cisasmallatomitformssingle,double,andtriplebondsitisintermediateinelectronegativity(2.5)itformsstrongbondswithC,H,O,N,andsomemetalsOrganicChemistryThestudyofSchematicViewofanAtomasmalldensenucleus,diameter10-14-10-15m,whichcontainspositivelychargedprotonsandmostofthemassoftheatomanextranuclearspace,diameter10-10m,whichcontainsnegativelychargedelectronsSchematicViewofanAtomasmaElectronConfigurationofAtomsElectronsareconfinedtoregionsofspacecalledprincipleenergylevels(shells)eachshellcanhold2n2electrons(n=1,2,3,4......)ElectronConfigurationofAtomElectronConfigurationofAtomsShellsaredividedintosubshellscalledorbitals,whicharedesignatedbytheletterss,p,d,f,........s(onepershell)p(setofthreepershell2andhigher)d(setoffivepershell3andhigher).....ElectronConfigurationofAtomElectronConfigurationofAtomsAufbauPrinciple:
orbitalsfillinorderofincreasingenergyfromlowestenergytohighestenergyPauliExclusionPrinciple:
onlytwoelectronscanoccupyanorbitalandtheirspinsmustbepairedHund’sRule:
whenorbitalsofequalenergyareavailablebuttherearenotenoughelectronstofillallofthem,oneelectronisaddedtoeachorbitalbeforeasecondelectronisaddedtoanyoneofthemElectronConfigurationofAtomElectronConfigurationofAtomsThepairingofelectronspinsElectronConfigurationofAtomElectronConfigurationofAtomsTable1.3TheGround-StateElectronConfigurationofElements1-18ElectronConfigurationofAtomLewisDotStructuresGilbertN.LewisValenceshell:
theoutermostoccupiedelectronshellofanatomValenceelectrons:
electronsinthevalenceshellofanatom;theseelectronsareusedtoformchemicalbondsandinchemicalreactionsLewisdotstructure:
thesymbolofanelementrepresentsthenucleusandallinnershellelectronsdotsrepresentvalenceelectronsLewisDotStructuresGilbertN.LewisDotStructuresTable1.4LewisDotStructuresforElements1-18LewisDotStructuresTable1.4LewisModelofBondingAtomsbondtogethersothateachatomacquiresanelectronconfigurationthesameasthatofthenoblegasnearestitinatomicnumberanatomthatgainselectronsbecomesananionanatomthatloseselectronsbecomesacationtheattractionofanionsandcationsleadstotheformationofionicsolidsanatommayshareelectronswithoneormoreatomstocompleteitsvalenceshell;achemicalbondformedbysharingelectronsiscalledacovalentbondbondsmaybepartiallyionicorpartiallycovalent;thesebondsarecalledpolarcovalentbondsLewisModelofBondingAtomsboElectronegativityElectronegativity:
ameasureofanatom’sattractionfortheelectronsitshareswithanotheratominachemicalbondPaulingscalegenerallyincreaseslefttorightinarowgenerallyincreasesbottomtotopinacolumnElectronegativityElectronegatiFormationofIonsAroughguideline:ionswillformifthedifferenceinelectronegativitybetweeninteractingatomsis1.9orgreaterexample:sodium(EN0.9)andfluorine(EN4.0)weuseasingle-headed(barbed)curvedarrowtoshowthetransferofoneelectronfromNatoFinformingNa+F-,thesingle3selectronfromNaistransferredtothepartiallyfilledvalenceshellofFFormationofIonsAroughguideCovalentBondsThesimplestcovalentbondisthatinH2thesingleelectronsfromeachatomcombinetoformanelectronpairthesharedpairfunctionsintwowayssimultaneously;itissharedbythetwoatomsandfillsthevalenceshellofeachatomThenumberofsharedpairsonesharedpairformsasinglebondtwosharedpairsformadoublebondthreesharedpairsformatriplebondCovalentBondsThesimplestcovPolarandNonpolarCovalentBondsAlthoughallcovalentbondsinvolvesharingofelectrons,theydifferwidelyinthedegreeofsharingWedividecovalentbondsintononpolarcovalentbondspolarcovalentbondsPolarandNonpolarCovalentBoPolarandNonpolarCovalentBondsanexampleofapolarcovalentbondisthatofH-ClthedifferenceinelectronegativitybetweenClandHis3.0-2.1=0.9weshowpolaritybyusingthesymbolsd+andd-,orbyusinganarrowwiththearrowheadpointingtowardthenegativeendandaplussignonthetailofthearrowatthepositiveendPolarandNonpolarCovalentBoPolarCovalentBondsBonddipolemoment(m):
ameasureofthepolarityofacovalentbondtheproductofthechargeoneitheratomofapolarbondtimesthedistancebetweenthenucleiTable1.7showsaveragebonddipolemomentsofselectedcovalentbondsPolarCovalentBondsBonddipolLewisStructuresTowriteaLewisstructuredeterminethenumberofvalenceelectronsdeterminethearrangementofatomsconnecttheatomsbysinglebondsarrangetheremainingelectronssothateachatomhasacompletevalenceshellshowabondingpairofelectronsasasinglelineshowanonbondingpairofelectronsasapairofdotsinasinglebondatomsshareonepairofelectrons,inadoublebondtheysharetwopairsofelectrons,andinatriplebondtheysharethreepairsofelectronsLewisStructuresTowriteaLewLewisStructures-Table1.3Inneutralmoleculeshydrogenhasonebondcarbonhas4bondsandnolonepairsnitrogenhas3bondsand1lonepairoxygenhas2bondsand2lonepairshalogenshave1bondand3lonepairsLewisStructures-Table1.3FormalChargeFormalcharge:thechargeonanatominamoleculeorapolyatomicionToderiveformalcharge1.writeacorrectLewisstructureforthemoleculeorion2.assigneachatomallitsunshared(nonbonding)electronsandone-halfitsshared(bonding)parethisnumberwiththenumberofvalenceelectronsintheneutral,unbondedatomFormalChargeFormalcharge:thFormalChargeExample:
DrawLewisstructures,andshowwhichatomineachbearstheformalchargeFormalChargeExample:DrawLewExceptionstotheOctetRuleMoleculescontainingatomsofGroup3Aelements,particularlyboronandaluminumAluminumchloride:::FBFFClAlClCl6electronsinthevalenceshellsofboronandaluminumBorontrifluoride:::::::::::::::ExceptionstotheOctetRuleMoExceptionstotheOctetRuleAtomsofthird-periodelementshave3dorbitalsandmayexpandtheirvalenceshellstocontainmorethan8electronsphosphorusmayhaveupto10ExceptionstotheOctetRuleAtExceptionstotheOctetRulesulfur,anotherthird-periodelement,formscompoundsinwhichitsvalenceshellcontains8,10,or12electronsExceptionstotheOctetRulesuFunctionalGroupsFunctionalgroup:anatomorgroupofatomswithinamoleculethatshowsacharacteristicsetofphysicalandchemicalpropertiesFunctionalgroupsareimportantforthreereason;theyare1.theunitsbywhichwedivideorganiccompoundsintoclasses2.thesitesofcharacteristicchemicalreactions3.thebasisfornamingorganiccompoundsFunctionalGroupsFunctionalgrAlcoholscontainan-OH(hydroxyl)groupEthanolmayalsobewrittenasacondensedstructuralformulaAlcoholscontainan-OH(hydroxAlcoholsalcoholsareclassifiedasprimary(1°),secondary(2°),ortertiary(3°)dependingonthenumberofcarbonatomsbondedtothecarbonbearingthe-OHgroupAlcoholsalcoholsareclassifieAlcoholstherearetwoalcoholswithmolecularformulaC3H8OAlcoholstherearetwoalcoholsAminescontainanaminogroup;ansp3-hybridizednitrogenbondedtoone,two,orthreecarbonatomsanaminemayby1°,2°,or3°CH3NHHCH3NHCH3CH3NCH3CH3Methylamine(a1°
amine)Dimethylamine(a2°
amine)Trimethylamine(a3°
amine):::Aminescontainanaminogroup;AldehydesandKetonescontainacarbonyl(C=O)groupAldehydesandKetonescontainaCarboxylicAcidscontainacarboxyl(-COOH)groupCarboxylicAcidscontainacarbCarboxylicEstersEster:aderivativeofacarboxylicacidinwhichthecarboxylhydrogenisreplacedbyacarbongroupCarboxylicEstersEster:aderiCarboxylicAmideCarboxylicamide,commonlyreferredtoasanamide:aderivativeofacarboxylicacidinwhichthe-OHofthe-COOHgroupisreplacedbyanaminethesixatomsoftheamidefunctionalgrouplieinaplanewithbondanglesofapproximately120°CarboxylicAmideCarboxylicamiVSEPRBasedonthetwinconceptsthatatomsaresurroundedbyregionsofelectrondensityregionsofelectrondensityrepeleachotherVSEPRBasedonthetwinconceptVSEPRModelExample:
predictallbondanglesforthesemoleculesandionsVSEPRModelExample:predictalPolarandNonpolarMoleculesTodetermineifamoleculeispolar,weneedtodetermineifthemoleculehaspolarbondsthearrangementofthesebondsinspaceMoleculardipolemoment():thevectorsumoftheindividualbonddipolemomentsinamoleculereportedindebyes(D)PolarandNonpolarMoleculesToPolarandNonpolarMoleculesthesemoleculeshavepolarbonds,buteachhasazerodipolemomentPolarandNonpolarMoleculesthPolarandNonpolarMoleculesthesemoleculeshavepolarbondsandarepolarmoleculesPolarandNonpol
温馨提示
- 1. 本站所有资源如无特殊说明,都需要本地电脑安装OFFICE2007和PDF阅读器。图纸软件为CAD,CAXA,PROE,UG,SolidWorks等.压缩文件请下载最新的WinRAR软件解压。
- 2. 本站的文档不包含任何第三方提供的附件图纸等,如果需要附件,请联系上传者。文件的所有权益归上传用户所有。
- 3. 本站RAR压缩包中若带图纸,网页内容里面会有图纸预览,若没有图纸预览就没有图纸。
- 4. 未经权益所有人同意不得将文件中的内容挪作商业或盈利用途。
- 5. 人人文库网仅提供信息存储空间,仅对用户上传内容的表现方式做保护处理,对用户上传分享的文档内容本身不做任何修改或编辑,并不能对任何下载内容负责。
- 6. 下载文件中如有侵权或不适当内容,请与我们联系,我们立即纠正。
- 7. 本站不保证下载资源的准确性、安全性和完整性, 同时也不承担用户因使用这些下载资源对自己和他人造成任何形式的伤害或损失。
最新文档
- 七年级道德与法治(上册)期中试卷及参考答案
- 2026年深圳中考地理答题技巧特训试卷(附答案可下载)
- 开展消防安全专项检查
- 2026重庆奉节县白帝镇人民政府招聘全日制公益性岗位人员3人备考题库含答案详解
- 内贸报价策略
- 切换效果介绍
- 分组介绍教学
- 小书包的变迁写物作文12篇
- 分级基金培训
- 分歧者介绍教学课件
- 公路工程施工安全技术与管理课件 第09讲 起重吊装
- 企业管理 华为会议接待全流程手册SOP
- 2026年城投公司笔试题目及答案
- 北京市东城区2025-2026学年高三上学期期末考试英语 有答案
- 2025年煤矿安全规程新增变化条款考试题库及答案
- 2025年教师师德师风自查问题清单及整改措施范文
- 2026年及未来5年市场数据中国激光干涉仪行业发展监测及投资战略规划研究报告
- 人工智能技术在小学语文阅读教学中的实践应用课题报告教学研究课题报告
- 2026年广东农垦火星农场有限公司公开招聘作业区管理人员备考题库及参考答案详解
- 国家安全生产十五五规划
- 河南省2025年普通高等学校对口招收中等职业学校毕业生考试语文试题 答案

评论
0/150
提交评论