



版权说明:本文档由用户提供并上传,收益归属内容提供方,若内容存在侵权,请进行举报或认领
文档简介
冻笔新诗懒写,寒炉美酒时温。醉看墨花月白,恍疑雪满前村。
——唐·李白《立冬》冻笔新诗懒写,寒炉美酒时温。Chapter6ReactionHeat,DirectionandExtentofChemicalReaction
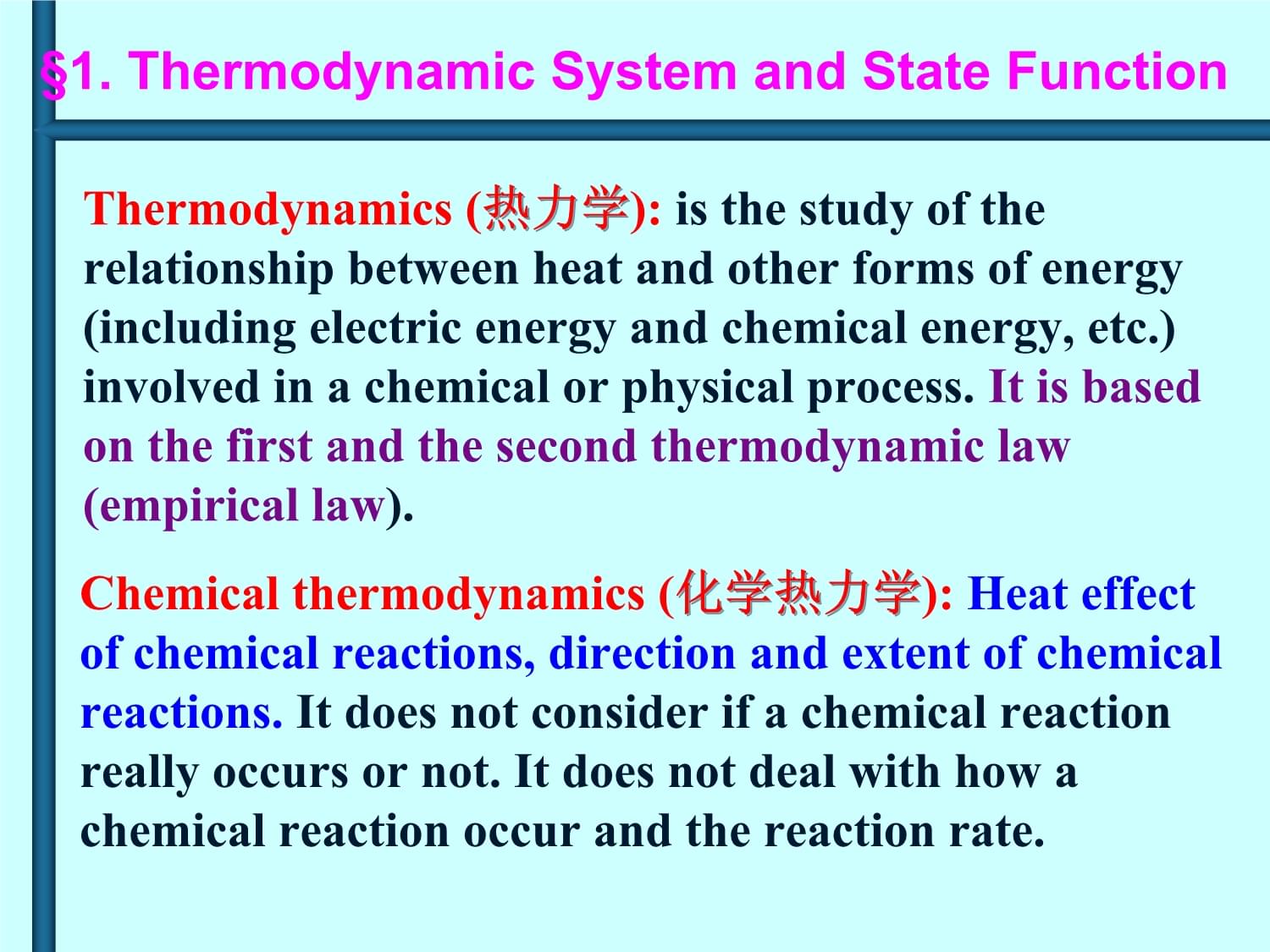
§1.Thermodynamicsystemandstatefunction§2.Energyconservationandheatofchemicalreaction§3.EntropyandGibbsfreeenergy§4.TheextentandequilibriumconstantofchemicalreactionsChapter6ReactionHeat,§1.§1.ThermodynamicSystemandStateFunctionThermodynamics(热力学):
isthestudyoftherelationshipbetweenheatandotherformsofenergy(includingelectricenergyandchemicalenergy,etc.)involvedinachemicalorphysicalprocess.Itisbasedonthefirstandthesecondthermodynamiclaw(empiricallaw).Chemicalthermodynamics(化学热力学):
Heateffectofchemicalreactions,directionandextentofchemicalreactions.Itdoesnotconsiderifachemicalreactionreallyoccursornot.Itdoesnotdealwithhowachemicalreactionoccurandthereactionrate.§1.ThermodynamicSystemandSI.SystemandsurroundingsSystem(系统):
tobeinvestigated.Thesubstanceormixtureofsubstancesunderstudyinwhichachangeoccursiscalledthethermodynamicsystem.Surroundings(环境):
directlyrelatedtosystem.Everythinginthevicinityofthethermodynamicsystem.§1.ThermodynamicSystemandStateFunctionI.SystemandsurroundingsSystOpensystem(开放系统):
canexchangematteraswellasenergywithitssurroundings.Closedsystem(封闭系统):
canexchangeenergybutnotmatterwithitssurroundings.Isolatedsystem(孤立系统):
canexchangeneitherenergynormatterwithitssurroundings.§1.ThermodynamicSystemandStateFunctionOpensystem(开放系统):canexchanII.Process(过程)Isothermal(等温):
Thetemperatureofthesystemremainsconstant,T=Tsurr=constant.Isobaric(等压):
Thepressureofthesystemremainsconstant,p=psurr=constant.Isochoric(等容):
Thevolumeofthesystemremainsconstant,V=constant.Adiabatic(绝热):
Noheatexchangewiththesurroundings,
Q=0.Cyclic(循环):
Finalstate=initialstate,U=0.§1.ThermodynamicSystemandStateFunction“Adiabatic”comesfromtheGreekwordsfor“notpassingthrough.”II.Process(过程)Isothermal(等温III.PropertiesofasystemMacroscopicpropertiesofasystem:Pressure,volume,temperature,mass,composition,….Extensiveproperties(广度性质):
proportionaltothequantity(moles).Example:mass,volume.§1.ThermodynamicSystemandStateFunctionIntensiveproperties(强度性质):
notdependentonthequantity(moles).Example:pressure,temperature,composition.III.PropertiesofasystemMacIV.StateandstatefunctionThestate
(状态)ofasystemisdefinedbyallpropertiesofthesystem.§1.ThermodynamicSystemandStateFunctionStateStatefunction(T,p,V,n) (U,H,S,G)Astatefunction(状态函数)isapropertyofasystem.Astatefunctiondependsonlyonitspresentstateandisindependentofanyprevioushistoryofthesystem.IV.StateandstatefunctionThStatefunction:extensiveandintensiveproperties.§1.ThermodynamicSystemandStateFunction(1)Theincrementofastatefunctionisonlydependentontheinitialandfinalstatesofthesystem,butnottheprocess.Statefunction:extensiveand373K1atm298K10atmIsobaricIsobaricIsothermalIsothermal298K1atminitialstates373K10atmfinal
states§1.ThermodynamicSystemandStateFunction373K298KIsobaricIsobaricIsot(2)Itisnotnecessarytolistoutallpropertiestodescribeastateofasystem.Forexample,thestateequationofidealgas:pV=nRT.p,V,Tn§1.ThermodynamicSystemandStateFunction(2)Itisnotnecessarytolis§1.ThermodynamicSystemandStateFunctionV.Internalenergy(内能)Energy:canbebrieflydefinedasthepotentialorcapacitytomovematter.Kineticenergy(动能):
theenergyassociatedwithanobjectbyvirtueofitsmotion.1.EnergyEnergycanexistindifferentforms,includingheat,light,electrical,andchemicalenergy,andthesedifferentformscanbeinterconverted.§1.ThermodynamicSystemandS§1.ThermodynamicSystemandStateFunctionTheSIunitofenergy,kg·m2·s-2,isgiventhenamejoule(焦耳,J).Thecalorie(卡路里,cal)isanon-SIunitofenergycommonlyused,originallydefinedastheamountofenergyrequiredtoraisethetemperatureofonegramofwaterbyonedegreeCelsius.(exactdefinition)§1.ThermodynamicSystemandS《基础化学》英文教学课件:chapter_69Calorie/1g4Calorie/1gCalorie(大卡)=1kilocalorie(千卡)=1000calorie(卡)
=4.184kJ9Calorie/1g4Calorie/1gCa§1.ThermodynamicSystemandStateFunctionPotentialenergy(势能):
theenergyanobjecthasbyvirtueofitspositioninafieldofforce.§1.ThermodynamicSystemandS§1.ThermodynamicSystemandStateFunctionInternalenergy(内能,U):Thesumofthekineticandpotentialenergiesoftheparticlesmakingupasystemisreferredtoastheinternalenergy.2.Internalenergy(U)ThewaterasawholehasEkandEp.However,H2Omoleculesaremadeupofsmallerparticles,electronsandnuclei.Eachoftheseparticlesalsohaskineticandpotentialenergy.U:extensiveproperty;absolutevalueofU?U:statefunction,U=(Ufinal-Uinitial)§1.ThermodynamicSystemandS§1.ThermodynamicSystemandStateFunctionThetotalenergyofaquantityofasubstanceequalsthesumofitskineticandpotentialenergiesasawholeplusitsinternalenergy.Normallywhenyoustudyasubstanceinthelab,thesubstanceisatrestinavessel.ItsEkasawholeiszero.Moreover,itsEpasawholeisconstantandcanbetakentobezero.Inthiscase,thetotalenergyofthesubstanceequalsitsinternalenergy,U.§1.ThermodynamicSystemandSVI.Heat(Q)andwork(W)Twoformsofenergyexchangebetweenasystemanditssurroundings.§1.ThermodynamicSystemandStateFunctionHeatflowsfromaregionofhighertemperaturetooneoflowertemperature;oncethetemperaturebecomeequal(thermalequilibrium),heatflowstops.Youwouldnotsaythatthesystemhasheat,becauseheatisonlyanenergyflow.Heat(热,Q)
isdefinedastheenergythatflowsintooroutofasystembecauseofadifferenceintemperaturebetweenthesystemanditssurroundings.
VI.Heat(Q)andwork(W)TwofWork(功,W)
istheenergyexchangethatresultswhenaforceFmovesanobjectthroughadistanced;workequalsW=F×d.§1.ThermodynamicSystemandStateFunctionHeatisabsorbedbythesystem:
(endothermic,吸热):Q>0;Heatisreleasedbythesystem:
(exothermic,放热):Q<0.Volumework(We);Electricwork,surfacework,….(Wf)Workdoneonthesystem:W>0;Workdonebythesystem:W<0Work(功,W)istheenergyexchaHeatandworkarenotstatefunctions,theyaredependentonthespecificprocess.§1.ThermodynamicSystemandStateFunctionHeatandworkarenotstatefuTTApoutlIsothermalexpansionofidealgas(理想气体的等温膨胀)
(pV=nRT)pinitial=405.2kPa,Vinitial=1.00L,Tinitial=273KInitialstate:pfinal=101.3kPa,Vfinal=4.00L,Tfinal=273KFinalstate:Example:§1.ThermodynamicSystemandStateFunctionTTApoutlIsothermalexpansionoTTApoutlIsothermalexpansionofidealgaspinitial=405.2kPaVinitial=1.00LTinitial=273Kpfinal=101.3kPaVfinal=4.00LTfinal=273Kpout=101.3kPa(1)p2=202.6kPaV2=2.00LTfinal=273K(2)pout=202.6kPa(I)pout=101.3kPa(II)Reversibleprocess(可逆过程)(3)§1.ThermodynamicSystemandStateFunctionTTApoutlIsothermalexpansiono(1) (2) TTApoutl§1.ThermodynamicSystemandStateFunction(1) (2) TTApoutl§1.ThermodynaW3>W2>W1§1.ThermodynamicSystemandStateFunctionHeatandworkarenotstatefunctions,theyaredependentonthespecificprocess.W3>W2>W1§1.ThermodyIsothermalexpansionofidealgasp1,V1p2,V2pVp1,V1p2,V2pVp1,V1p2,V2pV(1)(2)(3)Process(3)isareversible(可逆的)process.W3=Wmax
§1.ThermodynamicSystemandStateFunctionIsothermalexpansionofideal
§2.ConservationofEnergy&HeatofReactionI.Thefirstlawofthermodynamics(热力学第一定律)Thefirstlawofthermodynamicsstatesthattheinternalenergyofanisolatedsystemisconstant.Foraclosedsystem,themathematicalexpressionofthefirstlawofthermodynamics:
U=Q+WLater,W=We,Wf=0.Energycanneverbecreatedordestroyedinordinarychemicalreactionsandphysicalchanges,itcanonlytransformfromoneformtoanother.§2.ConservationofEnergy&
§2.ConservationofEnergy&HeatofReactionThissystemgainsinternalenergyfromtheheatabsorbed(165J)andlosesinternalenergyviatheworkdone(92J).Thusthenetchangeofinternalenergyis:§2.ConservationofEnergy&FritzHaber(1868-1934)NobelPrizeChemistry(1918)HaberProcess(哈伯循环)§2.ConservationofEnergy&HeatofReactionFritzHaberNobelPrizeChemist
§2.ConservationofEnergy&HeatofReactionII.Heatofreaction(反应热)Twocrystallinesubstances,Ba(OH)2·8H2OandNH4NO3,aremixedthoroughlyinaflask.Thentheflask,whichfeelsquitecoldtothetouch,issetinapuddleofwateronaboard.Inacoupleofminutes,theflaskandboardarefrozensolidlytogether.Theboardcanthenbeinvertedwiththeflaskfrozentoit.§2.ConservationofEnergy&
§2.ConservationofEnergy&HeatofReactionTheheatofreaction(atagiventemperature)isthevalueofQrequiredtoreturnasystemtothegiventemperatureatthecompletionofthereaction.(endothermic)1molBa(OH)2,(exothermic)1molCH4,§2.ConservationofEnergy&
§2.ConservationofEnergy&HeatofReactionMeasurementofheatofreaction(heretheheatofcombustionofgraphite).Bombcalorimeter(弹式量热计):adeviceusedtomeasuretheheatabsorbedorreleasedduringaphysicalorchemicalchange.§2.ConservationofEnergy&Biocalorimetry(生物量热学)微量量热技术
10203040t/h(1)ADSⅠE用肉胨培养的细菌特征热谱图(2)ADSⅡF用肉胨培养细菌26h后加抗生素的细菌特征热谱图§2.ConservationofEnergy&HeatofReactionBiocalorimetry(生物量热学)10III.Isochoricreactionheat(等容反应热)U=QV+W =QV+pV =QVConsiderachemicalreactionunderanisochoricconditions(Q=QV,V=0):U=QVTheheatofreactionatconstantvolume(isochoricreactionheat)equalsthechangeininternalenergyforthereaction.§2.ConservationofEnergy&HeatofReactionIII.Isochoricreactionheat(IV.Enthalpy(焓)andisobaricreactionheat(等压反应热)U=Ufinal–Uinitial=Qp+WConsiderachemicalreactionunderanisobaricconditions(Q=Qp):Forisobaricprocess:pinitial=pfinal=pout,then:(Ufinal+pfinalVfinal)–(Uinitial+pinitialVinitial)=QpHU+pV(definition)§2.ConservationofEnergy&HeatofReactionW=-poutV=-pout(Vfinal–Vinitial)IV.Enthalpy(焓)andisobaricrH=QpHfinal–Hinitial=Qp§2.ConservationofEnergy&HeatofReactionH:
enthalpy(焓),statefunction,extensiveproperties.Theheatofreactionatconstantpressure(isobaricreactionheat)equalsthechangeinenthalpyforthereaction.HU+pVH=QpHfinal–Hinitial=Qp§U=QVU=Q+WQV=Qp+WQV=Qp-PV(s.l,⊿n=0)QV=QpQV=Qp-PV(g)Qp=QV+nRTn=∑n(products)-∑n(reactants)§2.ConservationofEnergy&HeatofReactionU=QVU=Q+WQV=Qp+WQVExample:298K,1atm,2molH2and1molO2weremixed,and2molH2O(l)wasproducedafteracertaintime.Calculatetheextentofreactionofisochoricreactionheatandisobaricreactionheat,1molH2O(l)wasproduced
.2H2(g)+O2(g)=2H2O(l)Q=-571.6kJ·mol-1Solution:Qp=-285.8kJ·mol-11molH2O(l)wasproducedn=∑n(products)-∑n(reactants)=-3nRT=-7433J=-7.4kJQp=QV+nRT§2.ConservationofEnergy&HeatofReactionExample:298K,1atm,2molH2aQp=QV+nRTQV=Qp-nRT=-282.1
kJ·mol-1QV≈Qp1molH2O(l):nRT=-3.7kJ·mol-1Qp=-285.8kJ·mol-1H=QpU=QVH≈UQp=QV+nRTQV=Qp-nRT=§2.ConservationofEnergy&HeatofReactionThechangeinenthalpyforareactionatagiventemperatureandpressure(H,alsocalledtheenthalpyofreaction,反应焓)isobtainedbysubtractingtheenthalpyofthereactants(initialstate)fromtheenthalpyoftheproducts(finalstate).§2.ConservationofEnergy&HV.Extentofreaction(反应进度)Forareaction:B:reactantsorproducts;B:stoichiometriccoefficientofB;B(product)>0;B(reactant)<0.Extentofreactionξ(ksai):§2.ConservationofEnergy&HeatofReactionV.Extentofreaction(反应进度)FoExample6-1:10.0molH2and5.0molN2weremixed,and2.0molNH3wasproducedafteracertaintime.Calculatetheextentofreactionof(1)and(2).(1) (2)Solution:Accordingtoreaction(1):§2.ConservationofEnergy&HeatofReactionToproduce2.0molofNH3,1molofN2and3molofH2wereconsumed,respectively.Example6-1:10.0molH2and5Accordingtoreaction(2):§2.ConservationofEnergy&HeatofReactionExtentofreactionremainsthesamenomatterwhichreactantsorwhichproductsareusedtocalculateit.Extentofreactionisafunctionofstoichiometriccoefficientofreactantsandproducts.Accordingtoreaction(2):§2.VI.Thermochemicalequations(热化学方程)§2.ConservationofEnergy&HeatofReactionH:isobaricreactionheat(enthalpyofreaction);Definition:thechemicalequationforareaction(includingphaselabels)inwhichtheenthalpyofreaction(H)iswrittendirectlyaftertheequation.“r”:reaction;“m”:=1mol;:standardstate.VI.Thermochemicalequations(§2.ConservationofEnergy&HeatofReactionThetemperatureis298.15K(25ºC)ifotherwisestated.PureGas&PureLiquid:p=100kPa(1bar).Standardstate(标准态):Thestandardthermodynamicconditionschosenforsubstanceswhenlistingorcomparingthermodynamicdata:
standardpressurep(100kPa,1bar)andthe(any)specifiedtemperature(usually25ºC).Solute(insolution):p=100kPa,c=1mol·L-1.§2.ConservationofEnergy&H§2.ConservationofEnergy&HeatofReactionThestandardstateusedtobedefinedfor1atmratherthanfor1bar;however,thelatterisnowtheacceptedstandard.Thesmallchangeinstandardpressuremakesanegligibledifferencetomostnumericalvalues,soitisnormallysafetousetablesofdatacompiledfor1atm.Thisequationsaysthat,at25ºC,1molofhydrogengas(partialpressureof100kPa)reactswithone-halfmolofoxygengas(partialpressureof1bar)toproduce1molofliquidwater(partialpressureof1bar),and285.83kJofheatisreleased.§2.ConservationofEnergy&H§2.ConservationofEnergy&HeatofReaction①H2(g)+1/2O2(g)=H2O(l)Notes:1.Whenathermochemicalequationismultipliedbyanyfactor,thevalueofHforthenewequationisobtainedbymultiplyingthevalueofHintheoriginalequationbythesamefactor.2.Whenachemicalequationisreversed,thevalueofHisreversedinsign.③H2O(l)=1/2O2(g)+H2(g)②2H2(g)+O2(g)=2H2O(l)①H2(g)+1/2O2(g)=H2O(l)§2.ConservationofEnergy&H§2.ConservationofEnergy&HeatofReactionVII.Fuels(燃料)Afuelisanysubstancethatisburnedorsimilarlyreactedtoprovideheatandotherformsofenergy.Thehumanbodyrequiresaboutasmuchasenergyinadayasdoesa100-wattlightbulb.1.Foodsasfuels.Carbohydrate:Fat:§2.ConservationofEnergy&H§2.ConservationofEnergy&HeatofReactionPetroleumsupplieswillbe80%depletedatabouttheyear2030.Natural-gassuppliesmaybedepletedevensooner.Coalsuppliesaresufficienttolastseveralcenturies.Thisabundancehasspurredmuchresearchintodevelopingcommercialmethodsforconvertingcoaltomoreeasilyhandledliquidandgaseousfuels.2.Fossilfuels.SolidCoal(煤):Liquidpetroleum(石油):Naturalgas(天然气):§2.ConservationofEnergy&H§2.ConservationofEnergy&HeatofReaction3.Rocketfuels.ThefirststageoftheSaturnVlaunchvehicle(thatsentathree-manApollocrewtothemoon)used:Anunbelievable550tonsofkerosenewereburnedin2.5minutes(1.62×1011watts).Thesecondandthirdstageofliftoffusedliquidhydrogen(b.p.–253C)/oxygen(b.p.–183C)system:§2.ConservationofEnergy&H§2.ConservationofEnergy&HeatofReactionThelandingmodulefortheApollomissionusedafuelofhydrazineandanoxidizerofdinitrogentetroxide:Solidfuels(containingaluminummetalpowderand
othermaterialswithammoniumperchlorate
astheoxidizer)wereusedintheboosterrocketsoftheColumbiaspaceshuttle.Acloudofaluminumoxideformsastherocketsburn.§2.ConservationofEnergy&HVIII.Hess’slawHess’slawstatesthatforachemicalequationthatcanbewrittenasthesumoftwoormoresteps,theenthalpychangefortheoverallequationequalsthesumofenthalpychangesfortheindividualsteps.§2.ConservationofEnergy&HeatofReactionNomatterhowyougofromgivenreactantstoproducts,theenthalpychangefortheoverallchemicalchangeisthesame.reactantsproductsVIII.Hess’slawHess’slawsta§2.ConservationofEnergy&HeatofReaction§2.ConservationofEnergy&HⅨ.Calculatingtheisobaricreactionheat(enthalpyofreaction)1.Fromknownthermochemicalequations:Example6-2:(1)C(gra)+O2(g)=CO2(g)(2)CO(g)+½O2(g)=CO2(g)Calculatetheenthalpyofthefollowingreaction: (3)C(gra)+½O2(g)=CO(g).§2.ConservationofEnergy&HeatofReactionⅨ.CalculatingtheisobaricreSolution:§2.ConservationofEnergy&HeatofReactionC(gra)+O2(g)=CO2(g)CO(g)+½O2(g)=CO2(g)C(gra)+½O2(g)=CO(g)––Solution:§2.ConservationofEThermochemicalequationsfortheindividualstepsofareactionsequencemaybecombinedtogivethethermochemicalequationsfortheoverallreaction.§2.ConservationofEnergy&HeatofReactionC(gra)+O2(g)=CO2(g)CO(g)+½O2(g)=CO2(g)C(gra)+½O2(g)-CO(g)=0–C(gra)+½O2(g)=CO(g)CO(g)=C(gra)+½O2(g)Thermochemicalequationsfort2.FromstandardmolarenthalpiesofformationStandardmolarenthalpyofformation(标准摩尔生成焓)ofasubstance
istheenthalpychangefortheformationofonemoleofthesubstanceinitsstandardstatefromitselementsintheirmoststableformandintheirstandardstates.§2.ConservationofEnergy&HeatofReaction2.Fromstandardmolarenthalp§2.ConservationofEnergy&HeatofReactionAlthoughthereferenceformisusuallythestablestformofanelement,thechoiceisessentiallyarbitraryaslongasoneisconsistent.§2.ConservationofEnergy&HAllotropesofsulfurLeft:rhombicsulfur,thestableformoftheelementatroomtemperature.Right:Whenthissulfurismelted,thencooled,itformslongneedlesofmonoclinicsulfur,anotherallotrope.Atroomtemperature,monoclinicsulfurwillslowlychangebacktorhombicsulfur.Allotropesofsulfur《基础化学》英文教学课件:chapter_6elementsproductsreactants§2.ConservationofEnergy&HeatofReactionStandardmolarenthalpiesofformationcanbecombinedtoobtainthestandardenthalpyofanyreaction.isthemathematicalsymbolmeaning“thesumof”,andmandnarethecoefficientsofthesubstancesinthechemicalequation.elementsproductsreactants§2.C§2.ConservationofEnergy&HeatofReaction§2.ConservationofEnergy&H2NH3(g)+CO2(g)=CO(NH2)2(s)+H2O(l)Solution:Example6-3:Calculatethestandardreactionenthalpyofthefollowingreactionat298.15Kaccordingtodata.§2.ConservationofEnergy&HeatofReaction2NH3(g)+CO2(g)=CO(NH2)2(sWhatistheenthalpyofreaction,H,fortheformationoftungstencarbide,WC,fromtheelements?(Tungstencarbideisveryhardandisusedtomakecuttingtoolsandrockdrills.)Theenthalpychangeforthisreactionisdifficulttomeasuredirectly,becausethereactionoccursat1400ºC.However,theheatsofcombustionoftheelementsandoftungstencarbidecanbemeasuredeasily:Exercise:Whatistheenthalpyofreacti§2.ConservationofEnergy&HeatofReactionrHmisessentiallyconstantwithrespecttotemperature.Thisapproximationismostaccuratefortemperaturenottoodifferentfromthetemperature(25ºC)forwhichtherHmisobtained.Muchdifferenttemperaturesgivegreatererror.?§2.ConservationofEnergy&H3.FromstandardmolarenthalpiesofcombustionStandardmolarenthalpyofcombustion(标准摩尔燃烧焓)ofasubstanceistheenthalpychangeforthecompletecombustion(oxidation)ofonemoleofthesubstanceinitsstandardstatetoproductsintheirmoststableformandintheirstandardstates.§2.ConservationofEnergy&HeatofReaction3.FromstandardmolarenthalpreactantscombustionproductsproductsStandardmolarenthalpiesofcombustioncanbecombinedtoobtainthestandardenthalpyofanyreaction.§2.ConservationofEnergy&HeatofReactionreactantscombustionproductsprExample6-4:Calculatethestandardreactionenthalpyofthefollowingreactionat298.15Kaccordingtothestandardmolarenthalpyofcombustiondata.CH3CHO(l)+H2(g)=C2H5OH(l)Solution:§2.ConservationofEnergy&HeatofReactionExample6-4:Calculatethesta§3.EntropyandGibbsFreeEnergyI.Spontaneousprocess(自发过程)Somethingshappennaturally;somethingsdon’t.Waterflowsdownhillnaturally;wehavetopumpituphill.Heatflowsfromanhotobjecttoacoldone;arefrigeratorisneededtomakeheatflowfromacoldtoahotobject.Anironrustsinmoistair;butitrequireschemicalreactionstoconvertrusttoiron.§3.EntropyandGibbsFreeEne§3.EntropyandGibbsFreeEnergyI.Spontaneousprocess(自发过程)Aspontaneousprocessisaprocessthathasanaturaltendencytooccurwithoutbeingdrivenbyanexternalinfluence.Itisimportanttoappreciatethataspontaneouschangeneednotnecessarilytakeplaceatasignificantrate.Diamondshaveanaturaltendencytoturnintographite,butdiamondslastunchangedforcountlessyears----diamondsare,forpractice,forever.§3.EntropyandGibbsFreeEneKULeuvenAfdelingFotochemieenSpectroscopieProf.Dr.F.C.DeSchryverKULeuven71§3.EntropyandGibbsFreeEnergy(endothermic)Spontaneousreactionsmustbeexothermic(H<0)?§3.EntropyandGibbsFreeEneKULeuvenAfdelingFotochemieenSpectroscopieProf.Dr.F.C.DeSchryverKULeuven73II.Disorder(混乱度)andentropy(熵)Asingleideaaccountsforallspontaneouschange:Energyandmattertendtobecomemoredisordered.§3.EntropyandGibbsFreeEnergyEntropy,S,isathermodynamicquantitythatisameasureoftherandomnessordisorderinasystem.SIunit:J·K-1.Entropy:
statefunction:Itsquantityinagivenamountofsubstancedependsonlyonvariables(e.g,TandP)thatdeterminesthestateofthesubstance.1.EntropyII.Disorder(混乱度)andentropy§3.EntropyandGibbsFreeEnergy1moloficeat0ºCand100kPahasanentropyof41J·K-1.1molofliquidwaterat0ºCand100kPahasanentropyof63J·K-1.Forthemeltingoficetoliquidwater,Theentropyincreasesbecauseasubstancebecomesmoredisorderedwhenitmelts.§3.EntropyandGibbsFreeEne§3.EntropyandGibbsFreeEnergy2.Entropychangeforisothermalprocessesr:reversible.Whenasystemisatequilibrium,asmallchangeinaconditioncanmaketheprocessgoinonedirectionortheother.(热温商)§3.EntropyandGibbsFreeEneThethirdlawofthermodynamics:Asubstancethatisperfectlycrystallineat0Khasanentropyofzero.§3.EntropyandGibbsFreeEnergy3.ThethirdlawofthermodynamicsThethirdlawisthekeytoestimatetheentropy(conventionalentropy,规定熵)ofasubstanceatanytemperature,S(T),becauseittellsthatS(0)=0.Thethirdlawofthermodynamic§3.EntropyandGibbsFreeEnergy§3.EntropyandGibbsFreeEne§3.EntropyandGibbsFreeEnergy(1):Thestandardmolarentropyofagasishigherthanthatofthecorrespondingsolidandliquidatthesametemperature.(2):Standardmolarentropiesincreaseasthecomplexityofsubstancesincreases.Standardmolarentropy(标准摩尔熵),:Theconventionalentropyof1moleofsubstanceunderstandardstate(100kPa).Unit:J·K-1·mol-1.Homologousseries(同系物)§3.EntropyandGibbsFreeEne(3):Standardmolarentropiesincreasewiththetemperature.§3.EntropyandGibbsFreeEnergy(3):Standardmolarentropies§3.EntropyandGibbsFreeEnergyStandardmolarentropychangeforareaction(rSm):isthedifferenceinstandardmolarentropiesoftheproductsandreactants,takingintoaccounttheirstoichiometriccoefficients.Example6-5:Calculatethestandardmolarentropychange,rSm,at25ºCforthereactioninwhichureaisformedfromNH3andCO2.§3.EntropyandGibbsFreeEne§3.EntropyandGibbsFreeEnergySolution:2NH3(g)+CO2(g)NH2CONH2(aq)+H2O(l)§3.EntropyandGibbsFreeEne§3.EntropyandGibbsFreeEnergyTheentropyusuallyincreasesinthefollowingsituations:TopredicttheentropychangeforareactionAreactioninwhichamoleculeisbrokenintotwoormoresmallermolecules.Areactioninwhichthereisanincreaseinmolesofgas.Aprocessinwhichasolidchangestoliquidorgasoraliquidchangestoagas.§3.EntropyandGibbsFreeEne§3.EntropyandGibbsFreeEnergyExample6-6:Predictthesignoftheentropychangeofthefollowingreaction:Solution:Amoleculebreaksintosmallermolecules.Moreover,thisresultsinagasbeingreleased.Youpredictthattheentropyincreases.Inthisreaction,themolesofgasdecrease,whichwoulddecreasetheentropy.Youpredictthattheentropyshoulddecrease.Becausethereisnochangeinthenumberofmolesofgas,youcannotpredictthesignofentropychange.§3.EntropyandGibbsFreeEneIII.ThesecondlawofthermodynamicsTheentropyofanisolatedsystemincreaseinthecourseofanyspontaneousprocess.Theactualsystemanditssurro
温馨提示
- 1. 本站所有资源如无特殊说明,都需要本地电脑安装OFFICE2007和PDF阅读器。图纸软件为CAD,CAXA,PROE,UG,SolidWorks等.压缩文件请下载最新的WinRAR软件解压。
- 2. 本站的文档不包含任何第三方提供的附件图纸等,如果需要附件,请联系上传者。文件的所有权益归上传用户所有。
- 3. 本站RAR压缩包中若带图纸,网页内容里面会有图纸预览,若没有图纸预览就没有图纸。
- 4. 未经权益所有人同意不得将文件中的内容挪作商业或盈利用途。
- 5. 人人文库网仅提供信息存储空间,仅对用户上传内容的表现方式做保护处理,对用户上传分享的文档内容本身不做任何修改或编辑,并不能对任何下载内容负责。
- 6. 下载文件中如有侵权或不适当内容,请与我们联系,我们立即纠正。
- 7. 本站不保证下载资源的准确性、安全性和完整性, 同时也不承担用户因使用这些下载资源对自己和他人造成任何形式的伤害或损失。
最新文档
- 售后合同范本写
- 2024年光扫描数字化仪项目建议书
- 出租厂子厂房合同范本
- 商务运营培训合同范本
- 别墅出租免责合同
- 个人代理记账合同范本
- 公司间分成合同范本
- 再保险中文合同范本
- 成电路采购合同范本
- 整牙齿合同图
- 饲料猪油知识讲座
- 2024届北京市密云区物理高二下期末综合测试模拟试题含解析
- 护理美学修养讲课教案
- 2022-2023学年甘肃省普通高中高一学业水平合格考模拟历史试题(解析版)
- 双碳背景下零碳无废工厂实施路径及案例分析
- 2024年北京市燃气集团招聘笔试参考题库含答案解析
- 煤炭送货办法实施细则范文
- 湖南省长沙市长沙县2021-2022学年八年级下学期期末语文试题
- 腹痛患者护理查房
- 2023年《水利水电工程专业》职称考试试卷(中级工程师)(附答案)
- 餐券模板完整

评论
0/150
提交评论